当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective Multifunctional Aliphatic Amine Synthesis: Unraveling Universal Control Strategies in Catalytic Reductive Amination of Biobased α-Hydroxy Carbonyl Oxygenates
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-06-25 , DOI: 10.1021/acssuschemeng.4c02649 Benjamin Vermeeren 1 , Sofie Van Praet 1 , Axelle De Ridder 1 , Bert F. Sels 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-06-25 , DOI: 10.1021/acssuschemeng.4c02649 Benjamin Vermeeren 1 , Sofie Van Praet 1 , Axelle De Ridder 1 , Bert F. Sels 1
Affiliation
|
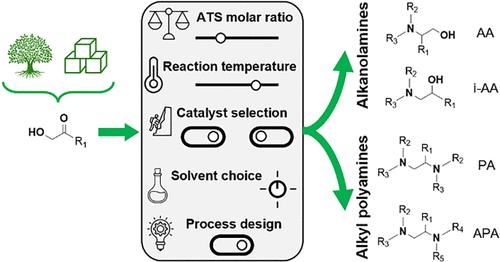
Catalytic reductive amination of carbohydrate-derived α-hydroxy carbonyl oxygenates offers an elegant approach to access both aliphatic alkanolamines and alkyl polyamines via the same reaction pathway. However, the advantage of producing these distinct aliphatic amine products from a single feedstock is accompanied by the challenge of product selectivity. This work therefore focused on exploring asymmetrically substituted α-hydroxy carbonyl oxygenates as an uncharted, yet potentially valuable subgroup of substrates. Here, the selectivity challenge was tackled by comprehensively assessing the effectiveness of multiple control handles based on the underlying reaction mechanism. These handles ranged from reaction parameters such as (co-)catalyst selection, reaction temperature, amine-to-substrate molar ratio, and solvent choice to process design, encompassing one-step and one-pot-two-step approaches. By synergistically combining the most decisive handles, we distilled three distinct, universally applicable and highly selective control strategies. Each strategy was tailored to regulate the formation of either one of the two alkanolamine isomers or the polyamine product. As a proof of concept, a strategy was proposed, inspired by the polyamine-tailored strategy, favoring the formation of high-value asymmetrically substituted alkyl polyamines. This proof of concept underscored the importance of both the relative reactivity of the two amine reactants and the stability of the formed α-amino ketone intermediate.
中文翻译:

选择性多功能脂肪胺合成:揭示生物基 α-羟基羰基含氧化合物催化还原胺化的通用控制策略
碳水化合物衍生的 α-羟基羰基含氧化合物的催化还原胺化提供了一种通过相同反应途径获得脂肪族烷醇胺和烷基多胺的优雅方法。然而,从单一原料生产这些不同的脂肪胺产品的优势也伴随着产品选择性的挑战。因此,这项工作的重点是探索不对称取代的 α-羟基羰基含氧化合物作为未知但具有潜在价值的底物亚组。在这里,通过基于潜在反应机制综合评估多个控制手柄的有效性来解决选择性挑战。这些处理范围从反应参数(例如(共)催化剂选择、反应温度、胺与底物摩尔比、溶剂选择到工艺设计),包括一步法和一锅两步法。通过协同组合最具决定性的手柄,我们提炼出三种不同的、普遍适用的和高度选择性的控制策略。每种策略都经过专门设计,以调节两种烷醇胺异构体之一或多胺产物的形成。作为概念验证,受多胺定制策略的启发,提出了一种策略,有利于形成高价值的不对称取代烷基多胺。这一概念验证强调了两种胺反应物的相对反应性和所形成的 α-氨基酮中间体的稳定性的重要性。
更新日期:2024-06-25
中文翻译:

选择性多功能脂肪胺合成:揭示生物基 α-羟基羰基含氧化合物催化还原胺化的通用控制策略
碳水化合物衍生的 α-羟基羰基含氧化合物的催化还原胺化提供了一种通过相同反应途径获得脂肪族烷醇胺和烷基多胺的优雅方法。然而,从单一原料生产这些不同的脂肪胺产品的优势也伴随着产品选择性的挑战。因此,这项工作的重点是探索不对称取代的 α-羟基羰基含氧化合物作为未知但具有潜在价值的底物亚组。在这里,通过基于潜在反应机制综合评估多个控制手柄的有效性来解决选择性挑战。这些处理范围从反应参数(例如(共)催化剂选择、反应温度、胺与底物摩尔比、溶剂选择到工艺设计),包括一步法和一锅两步法。通过协同组合最具决定性的手柄,我们提炼出三种不同的、普遍适用的和高度选择性的控制策略。每种策略都经过专门设计,以调节两种烷醇胺异构体之一或多胺产物的形成。作为概念验证,受多胺定制策略的启发,提出了一种策略,有利于形成高价值的不对称取代烷基多胺。这一概念验证强调了两种胺反应物的相对反应性和所形成的 α-氨基酮中间体的稳定性的重要性。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号