当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A novel approach to the tetracyclic frameworks of anislactone-type sesquiterpenoids
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-26 , DOI: 10.1039/d4qo00915k Long He 1 , Xiaocheng Zhang 1 , Sen Li 1 , Xuexue Tian 1 , Yimeng Han 1 , Haitao Xie 2 , Weiqing Xie 1, 3
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-26 , DOI: 10.1039/d4qo00915k Long He 1 , Xiaocheng Zhang 1 , Sen Li 1 , Xuexue Tian 1 , Yimeng Han 1 , Haitao Xie 2 , Weiqing Xie 1, 3
Affiliation
|
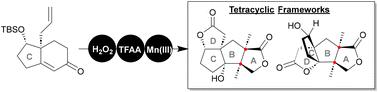
Herein, we present a novel approach to construct the A-B-C-D ring system of anislactone-type sesquiterpenoids. This innovative strategy involves a H2O2-mediated oxidative ring contraction reaction to form a 5/5 fused skeleton, while simultaneously generating contiguous quaternary carbon centers (CQCCs). Additionally, γ-lactones (A- and D-rings) are assembled through Pummerer reaction initiated cyclization and sequential Mukaiyama hydration/translactonization, respectively. Despite our efforts to introduce a hydroxyl group at C7 via C–H oxidation, these endeavors ultimately proved unsuccessful.
中文翻译:

茴香内酯型倍半萜四环骨架的新方法
在此,我们提出了一种构建茴香内酯型倍半萜类化合物 A-B-C-D 环系统的新方法。这种创新策略涉及 H 2 O 2 介导的氧化环收缩反应,形成 5/5 融合骨架,同时生成连续的季碳中心 (CQCC)。此外,γ-内酯(A-环和D-环)分别通过Pummerer反应引发的环化和连续的Mukaiyama水合/反内酯化来组装。尽管我们努力通过 C-H 氧化在 C7 上引入羟基,但这些努力最终被证明是不成功的。
更新日期:2024-06-26
中文翻译:

茴香内酯型倍半萜四环骨架的新方法
在此,我们提出了一种构建茴香内酯型倍半萜类化合物 A-B-C-D 环系统的新方法。这种创新策略涉及 H 2 O 2 介导的氧化环收缩反应,形成 5/5 融合骨架,同时生成连续的季碳中心 (CQCC)。此外,γ-内酯(A-环和D-环)分别通过Pummerer反应引发的环化和连续的Mukaiyama水合/反内酯化来组装。尽管我们努力通过 C-H 氧化在 C7 上引入羟基,但这些努力最终被证明是不成功的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号