当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A copper-catalyzed annulation reaction of diazo esters with propargyl amines for the synthesis of 2,5-dihydropyrroles
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-26 , DOI: 10.1039/d4qo00751d Haodong Xie 1 , Wei Zhao 2 , Heping Wei 2 , Yang Yu 3 , Lili Zhao 4 , Jonathan B. Baell 1 , Fei Huang 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-26 , DOI: 10.1039/d4qo00751d Haodong Xie 1 , Wei Zhao 2 , Heping Wei 2 , Yang Yu 3 , Lili Zhao 4 , Jonathan B. Baell 1 , Fei Huang 1
Affiliation
|
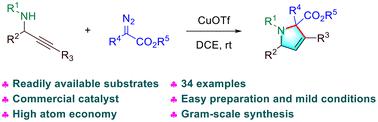
2,5-Dihydropyrrole derivatives are considered privileged structures as reflected by their presence in many biologically active molecules and therapeutic agents. Herein, we develop a concise and efficient method utilizing a commercially available copper salt as a catalyst to facilitate the formation of 2,5-dihydropyrroles from propargyl amines and diazo esters. This reaction proceeds via the formation of an N-ylide, followed by bond cleavage/recombination. Control experiments and density functional theory (DFT) calculations provide a detailed analysis of the reaction pathway. The key features of this protocol include simple operation, readily available starting materials, mild conditions, and high atom economy. In addition, the gram-scale preparation experiment and the transformations of 2,5-dihydropyrroles demonstrate the potential applicability of our synthetic method.
中文翻译:

铜催化重氮酯与炔丙胺的成环反应合成 2,5-二氢吡咯
2,5-二氢吡咯衍生物被认为是特殊结构,这反映在它们存在于许多生物活性分子和治疗剂中。在此,我们开发了一种简洁有效的方法,利用市售的铜盐作为催化剂,促进由炔丙胺和重氮酯形成2,5-二氢吡咯。该反应通过形成 N-叶立德,然后进行键断裂/重组来进行。控制实验和密度泛函理论 (DFT) 计算提供了反应途径的详细分析。该方案的主要特点包括操作简单、起始原料易得、条件温和、原子经济性高。此外,克级制备实验和2,5-二氢吡咯的转化证明了我们的合成方法的潜在适用性。
更新日期:2024-06-26
中文翻译:

铜催化重氮酯与炔丙胺的成环反应合成 2,5-二氢吡咯
2,5-二氢吡咯衍生物被认为是特殊结构,这反映在它们存在于许多生物活性分子和治疗剂中。在此,我们开发了一种简洁有效的方法,利用市售的铜盐作为催化剂,促进由炔丙胺和重氮酯形成2,5-二氢吡咯。该反应通过形成 N-叶立德,然后进行键断裂/重组来进行。控制实验和密度泛函理论 (DFT) 计算提供了反应途径的详细分析。该方案的主要特点包括操作简单、起始原料易得、条件温和、原子经济性高。此外,克级制备实验和2,5-二氢吡咯的转化证明了我们的合成方法的潜在适用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号