当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into the mechanism and origin of solvent-controlled chemodivergence in the synthesis of Au-catalyzed bicyclo[2.2.1]heptanes from 3-alkoxy-1,6-diynes: a DFT perspective
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-25 , DOI: 10.1039/d4qo00816b Aili Feng 1 , Dongju Zhang 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-25 , DOI: 10.1039/d4qo00816b Aili Feng 1 , Dongju Zhang 1
Affiliation
|
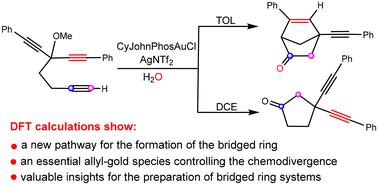
Density functional theory (DFT) calculations reveal a distinct mechanism for the Au(I)-catalyzed synthesis of bicyclo-[2.2.1]heptanes through cycloisomerizations of 3-alkoxyl-1,6-diynes. The proposed mechanism highlights the crucial involvement of an allyl-gold species and rationalizes all experimental observations, including the solvent-controlled chemodivergence of the reaction. Specifically, in the less polar toluene solvent, the reaction involves a key allyl-gold species with an energy barrier of 24.1 kcal mol−1, leading to the bridged ring product. Conversely, in the polar 1,2-dichloroethane solvent, the reaction occurs via an alkyl-gold species with an energy barrier of 29.2 kcal mol−1, delivering the monocyclic product. This new reactivity concept could potentially assist the exploration of new and unconventional strategies for the preparation of bridged ring systems.
中文翻译:

从 3-烷氧基-1,6-二炔合成 Au 催化双环[2.2.1]庚烷中溶剂控制化学发散的机制和起源的见解:DFT 视角
密度泛函理论 (DFT) 计算揭示了 Au() 催化通过 3-烷氧基-1,6-二炔环异构化合成双环-[2.2.1]庚烷的独特机制。所提出的机制强调了烯丙基金物质的重要参与,并使所有实验观察结果合理化,包括溶剂控制的反应化学发散。具体来说,在极性较小的甲苯溶剂中,反应涉及能垒为 24.1 kcal mol −1 的关键烯丙基金物质,从而产生桥环产物。相反,在极性 1,2-二氯乙烷溶剂中,反应通过能垒为 29.2 kcal mol −1 的烷基金物质发生,生成单环产物。这种新的反应性概念可能有助于探索制备桥环系统的新的非常规策略。
更新日期:2024-06-27
中文翻译:

从 3-烷氧基-1,6-二炔合成 Au 催化双环[2.2.1]庚烷中溶剂控制化学发散的机制和起源的见解:DFT 视角
密度泛函理论 (DFT) 计算揭示了 Au() 催化通过 3-烷氧基-1,6-二炔环异构化合成双环-[2.2.1]庚烷的独特机制。所提出的机制强调了烯丙基金物质的重要参与,并使所有实验观察结果合理化,包括溶剂控制的反应化学发散。具体来说,在极性较小的甲苯溶剂中,反应涉及能垒为 24.1 kcal mol −1 的关键烯丙基金物质,从而产生桥环产物。相反,在极性 1,2-二氯乙烷溶剂中,反应通过能垒为 29.2 kcal mol −1 的烷基金物质发生,生成单环产物。这种新的反应性概念可能有助于探索制备桥环系统的新的非常规策略。






































 京公网安备 11010802027423号
京公网安备 11010802027423号