当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nickel-catalyzed arylative cyclization of 1,6-enynes: arylation of unactivated alkene moieties
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-25 , DOI: 10.1039/d4qo00784k Jian Gao 1 , Qi Wei 2 , Zeqing Zhang 1 , Zhishan Su 2 , Jialin Ming 1 , Yongmin Zhang 1, 3
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-25 , DOI: 10.1039/d4qo00784k Jian Gao 1 , Qi Wei 2 , Zeqing Zhang 1 , Zhishan Su 2 , Jialin Ming 1 , Yongmin Zhang 1, 3
Affiliation
|
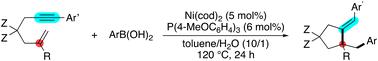
We developed catalytic cyclization of 1,6-enynes, whereby an aryl group is introduced at an unactivated alkene moiety. The reaction of a 1,6-enyne with an arylboronic acid in the presence of an Ni(cod)2/P(4-MeOC6H4)3 complex yields a five-membered ring product incorporating an all-carbon quaternary center. Experimental studies and extensive DFT calculations reveal that an Ni(I) species is involved in the catalytic cycle, which is an uncommon pathway involving transmetalation, oxidative cyclization, selective sp3–sp2 carbon–carbon bond formation, and finally hydrolysis.
中文翻译:

镍催化的 1,6-烯炔芳基化环化:未活化烯烃部分的芳基化
我们开发了 1,6-烯炔的催化环化,从而在未活化的烯烃部分引入芳基。 1,6-烯炔与芳基硼酸在 Ni(cod) 2 /P(4-MeOC 6 H 4 存在下的反应) 3 配合物产生包含全碳四元中心的五元环产物。实验研究和广泛的 DFT 计算表明,Ni() 物种参与催化循环,这是一种不常见的途径,涉及金属转移、氧化环化、选择性 sp 3 –sp 2 碳–碳键形成,最后水解。
更新日期:2024-06-27
中文翻译:

镍催化的 1,6-烯炔芳基化环化:未活化烯烃部分的芳基化
我们开发了 1,6-烯炔的催化环化,从而在未活化的烯烃部分引入芳基。 1,6-烯炔与芳基硼酸在 Ni(cod) 2 /P(4-MeOC 6 H 4 存在下的反应) 3 配合物产生包含全碳四元中心的五元环产物。实验研究和广泛的 DFT 计算表明,Ni() 物种参与催化循环,这是一种不常见的途径,涉及金属转移、氧化环化、选择性 sp 3 –sp 2 碳–碳键形成,最后水解。






































 京公网安备 11010802027423号
京公网安备 11010802027423号