当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemoselective reaction of methoxyaminomethyl BODIPYs with unprotected carbohydrates: a powerful tool for accessing BODIPY neoglycosides
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-25 , DOI: 10.1039/d4qo00886c Ana M. Gómez 1 , Luis García-Fernández 2, 3 , Andrés G. Santana 1 , Clara Uriel 1 , Leire Gartzia-Rivero 4 , Jorge Bañuelos 4 , Inmaculada Garcia-Moreno 5 , Lourdes Infantes 6 , María Rosa Aguilar 2, 3 , J. Cristobal Lopez 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-25 , DOI: 10.1039/d4qo00886c Ana M. Gómez 1 , Luis García-Fernández 2, 3 , Andrés G. Santana 1 , Clara Uriel 1 , Leire Gartzia-Rivero 4 , Jorge Bañuelos 4 , Inmaculada Garcia-Moreno 5 , Lourdes Infantes 6 , María Rosa Aguilar 2, 3 , J. Cristobal Lopez 1
Affiliation
|
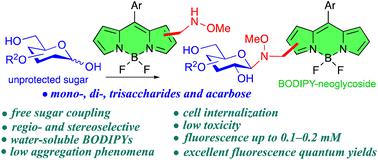
The neoglycosylation of methoxyaminomethyl-appended BODIPYs with unprotected reducing mono-, di-, and trisaccharides produces, in a regio- and stereoselective manner, cyclic N-glycosyl-N-methoxy–BODIPY conjugates, as a relevant class of neoglycosides that display excellent photophysical characteristics in pure water, even at high dye concentrations. In addition, the cellular uptake of some of the neoglycosylated BODIPYs has been confirmed via fluorescence microscopy, and a BODIPY–acarbose conjugate showed comparable enzymatic inhibitory activity to acarbose for two different α-amylases: A. oryzae α-amylase (AOA) and human salivary α-amylase (HSA).
中文翻译:

甲氧基氨基甲基 BODIPY 与未受保护的碳水化合物的化学选择性反应:获取 BODIPY 新糖苷的强大工具
甲氧基氨基甲基附加的 BODIPY 与未保护的还原性单糖、二糖和三糖的新糖基化以区域和立体选择性方式产生环状 N-糖基-N-甲氧基-BODIPY 缀合物,作为一类相关的新糖苷,具有优异的光物理性能纯水中的特性,即使在高染料浓度下也是如此。此外,一些新糖基化 BODIPY 的细胞摄取已通过荧光显微镜得到证实,对于两种不同的 α-淀粉酶:米曲霉 α-淀粉酶 (AOA) 和人,BODIPY-阿卡波糖缀合物显示出与阿卡波糖相当的酶抑制活性。唾液α-淀粉酶(HSA)。
更新日期:2024-06-27
中文翻译:

甲氧基氨基甲基 BODIPY 与未受保护的碳水化合物的化学选择性反应:获取 BODIPY 新糖苷的强大工具
甲氧基氨基甲基附加的 BODIPY 与未保护的还原性单糖、二糖和三糖的新糖基化以区域和立体选择性方式产生环状 N-糖基-N-甲氧基-BODIPY 缀合物,作为一类相关的新糖苷,具有优异的光物理性能纯水中的特性,即使在高染料浓度下也是如此。此外,一些新糖基化 BODIPY 的细胞摄取已通过荧光显微镜得到证实,对于两种不同的 α-淀粉酶:米曲霉 α-淀粉酶 (AOA) 和人,BODIPY-阿卡波糖缀合物显示出与阿卡波糖相当的酶抑制活性。唾液α-淀粉酶(HSA)。






































 京公网安备 11010802027423号
京公网安备 11010802027423号