当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereoselective strain-release Ferrier rearrangement: the dual role of catalysts
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-25 , DOI: 10.1039/d4qo00410h Huajun Zhang 1 , Aoxin Guo 1 , Han Ding 1 , Yuan Xu 1 , Yuhan Zhang 1 , Dan Yang 1 , Xue-Wei Liu 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-25 , DOI: 10.1039/d4qo00410h Huajun Zhang 1 , Aoxin Guo 1 , Han Ding 1 , Yuan Xu 1 , Yuhan Zhang 1 , Dan Yang 1 , Xue-Wei Liu 1
Affiliation
|
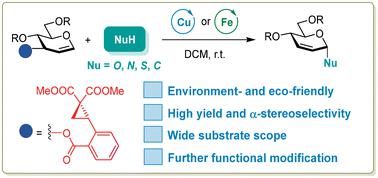
Herein, we report an efficient, stereoselective synthesis of 2,3-unsaturated glycosides under mild conditions through a novel Ferrier rearrangement of reasonably designed glycal donors with C3-position ortho-2,2-dimethoxycarbonycyclopropylbenzoyl (CCBz), facilitated by user-friendly, eco- and environmental-friendly Cu(OTf)2 or Fe(OTf)3. The newly devised rearrangement tactic is highly α-stereoselective and applies to a broad scope of nucleophile acceptors, enabling the construction of 2,3-unsaturated O-, S-, N-, and C-glycosides, with exceptional yields and stereoselectivities. DFT calculations were conducted to elucidate the reaction mechanism, unveiling the dual role of the metal catalysts in activating the glycal donor through promoting ring-opening of the intramolecularly incorporated donor–acceptor cyclopropane (DAC) of CCBz and directing the following α-face-preferential nucleophilic attack of the incoming acceptor mediated by H-bond interactions.
中文翻译:

立体选择性应变释放费里尔重排:催化剂的双重作用
在此,我们报告了在温和条件下,通过合理设计的糖基供体与C3位邻-2,2-二甲氧基羰基环丙基苯甲酰基(CCBz)的新型费里尔重排,在用户友好的促进下,高效、立体选择性地合成2,3-不饱和糖苷。生态环保的Cu(OTf) 2 或Fe(OTf) 3 。新设计的重排策略具有高度的α-立体选择性,适用于广泛的亲核受体,能够构建2,3-不饱和O-、S-、N-和C-糖苷,具有优异的产率和立体选择性。通过DFT计算阐明了反应机理,揭示了金属催化剂通过促进CCBz分子内结合的供体-受体环丙烷(DAC)开环来激活糖醛供体的双重作用,并指导以下α-面优先反应由氢键相互作用介导的传入受体的亲核攻击。
更新日期:2024-06-26
中文翻译:

立体选择性应变释放费里尔重排:催化剂的双重作用
在此,我们报告了在温和条件下,通过合理设计的糖基供体与C3位邻-2,2-二甲氧基羰基环丙基苯甲酰基(CCBz)的新型费里尔重排,在用户友好的促进下,高效、立体选择性地合成2,3-不饱和糖苷。生态环保的Cu(OTf) 2 或Fe(OTf) 3 。新设计的重排策略具有高度的α-立体选择性,适用于广泛的亲核受体,能够构建2,3-不饱和O-、S-、N-和C-糖苷,具有优异的产率和立体选择性。通过DFT计算阐明了反应机理,揭示了金属催化剂通过促进CCBz分子内结合的供体-受体环丙烷(DAC)开环来激活糖醛供体的双重作用,并指导以下α-面优先反应由氢键相互作用介导的传入受体的亲核攻击。











































 京公网安备 11010802027423号
京公网安备 11010802027423号