当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
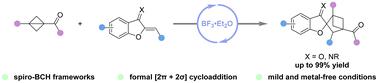
Access to spiro-bicyclo[2.1.1]hexanes via BF3·Et2O-catalyzed formal [2π + 2σ] cycloaddition of bicyclo[1.1.0]butanes with benzofuran-derived oxa(aza)dienes
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-25 , DOI: 10.1039/d4qo00511b Wei-Ping Deng , Jia-Yi Su , Jian Zhang , Zhiyun Xin , Hao Li , Hanliang Zheng
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-25 , DOI: 10.1039/d4qo00511b Wei-Ping Deng , Jia-Yi Su , Jian Zhang , Zhiyun Xin , Hao Li , Hanliang Zheng
|
Herein, we have developed a method for the construction of spiro[benzofuran-2,2′-bicyclo[2.1.1]hexanes] via BF3·Et2O-catalyzed formal [2π + 2σ] cycloaddition of bicyclo[1.1.0]butanes with benzofuran-derived oxa(aza)dienes. This transformation allowed for facile access to a variety of functionalized spiro-bicyclo[2.1.1]hexanes in good yields (up to 99% yield) with excellent chemoselectivities and a broad substrate scope (34 examples) under mild reaction conditions. Moreover, the synthetic utility of the cycloadducts was further emphasized through a scale-up experiment and subsequent synthetic transformations.
中文翻译:

通过 BF3·Et2O 催化双环[1.1.0]丁烷与苯并呋喃衍生的氧杂(氮杂)二烯的形式[2π + 2σ]环加成反应获得螺双环[2.1.1]己烷
在此,我们开发了一种通过 BF 3 ·Et 2 O 催化的缩甲醛构建螺[苯并呋喃-2,2′-双环[2.1.1]己烷]的方法双环[1.1.0]丁烷与苯并呋喃衍生的氧杂(氮杂)二烯的[2π + 2σ]环加成反应。这种转化允许在温和的反应条件下以良好的收率(高达 99% 收率)轻松获得各种官能化螺环双环[2.1.1]己烷,并具有优异的化学选择性和广泛的底物范围(34 个实例)。此外,通过放大实验和随后的合成转化,进一步强调了环加合物的合成效用。
更新日期:2024-06-25
中文翻译:

通过 BF3·Et2O 催化双环[1.1.0]丁烷与苯并呋喃衍生的氧杂(氮杂)二烯的形式[2π + 2σ]环加成反应获得螺双环[2.1.1]己烷
在此,我们开发了一种通过 BF 3 ·Et 2 O 催化的缩甲醛构建螺[苯并呋喃-2,2′-双环[2.1.1]己烷]的方法双环[1.1.0]丁烷与苯并呋喃衍生的氧杂(氮杂)二烯的[2π + 2σ]环加成反应。这种转化允许在温和的反应条件下以良好的收率(高达 99% 收率)轻松获得各种官能化螺环双环[2.1.1]己烷,并具有优异的化学选择性和广泛的底物范围(34 个实例)。此外,通过放大实验和随后的合成转化,进一步强调了环加合物的合成效用。






































 京公网安备 11010802027423号
京公网安备 11010802027423号