当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
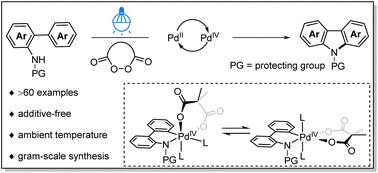
Palladium(II)-catalyzed intramolecular C–H amination to carbazole: the crucial role of cyclic diacyl peroxides
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-25 , DOI: 10.1039/d4qo00779d Ting Yuan , Kang Fu , Lei Shi
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-25 , DOI: 10.1039/d4qo00779d Ting Yuan , Kang Fu , Lei Shi
|
Despite extensive efforts in metal-free synthesis, strained cyclic diacyl peroxides have not been invoked as external oxidants in high-oxidation state palladium-catalyzed reactions and their involvement in the corresponding elementary steps has not been studied. Herein, a PdII-catalyzed intramolecular oxidative C–H amination has been successfully developed for the construction of carbazole architectures under additive-free and mild conditions. The presented method proved to be compatible with a variety of functional groups and scalable synthesis, which enabled the late-stage diversification of bioactive molecules and facilitated the concise syntheses of carbazole alkaloid derivatives. Importantly, the use of strained cyclic diacyl peroxides as a sacrificial oxidant is critical for controlling the reactivity of high-valent Pd intermediates and their selectivity to multiple competing reductive elimination events, owing to the rigid constraint imposed by the bidentate dicarboxylate ligand. Mechanistic studies suggested that this reaction proceeds via a unique PdII/PdIV catalytic pathway, and the turnover-limiting step is substrate coordination to the palladium catalyst. Moreover, photophysical investigation showed that varying N-protecting groups and increasing conjugated systems had a clear influence on the photophysical properties of carbazole derivatives. The calculation of six green metrics demonstrates the latent greenness of this catalytic system.
中文翻译:

钯(II)催化分子内C-H胺化生成咔唑:环状二酰基过氧化物的关键作用
尽管在无金属合成方面做出了广泛的努力,但应变环状二酰基过氧化物尚未被用作高氧化态钯催化反应中的外部氧化剂,并且尚未研究它们在相应基本步骤中的参与。在此,我们成功开发了Pd II 催化的分子内氧化C-H胺化,用于在无添加剂和温和的条件下构建咔唑结构。该方法被证明能够兼容多种官能团和可规模化合成,从而实现了生物活性分子的后期多样化,并促进了咔唑生物碱衍生物的简洁合成。重要的是,由于二齿二羧酸配体施加的严格约束,使用应变环状二酰基过氧化物作为牺牲氧化剂对于控制高价钯中间体的反应性及其对多个竞争性还原消除事件的选择性至关重要。机理研究表明该反应通过独特的Pd II /Pd IV 催化途径进行,并且转换限制步骤是底物与钯催化剂的配位。此外,光物理研究表明,不同的N-保护基团和增加的共轭体系对咔唑衍生物的光物理性质有明显的影响。六个绿色指标的计算证明了该催化系统的潜在绿色性。
更新日期:2024-06-25
中文翻译:

钯(II)催化分子内C-H胺化生成咔唑:环状二酰基过氧化物的关键作用
尽管在无金属合成方面做出了广泛的努力,但应变环状二酰基过氧化物尚未被用作高氧化态钯催化反应中的外部氧化剂,并且尚未研究它们在相应基本步骤中的参与。在此,我们成功开发了Pd II 催化的分子内氧化C-H胺化,用于在无添加剂和温和的条件下构建咔唑结构。该方法被证明能够兼容多种官能团和可规模化合成,从而实现了生物活性分子的后期多样化,并促进了咔唑生物碱衍生物的简洁合成。重要的是,由于二齿二羧酸配体施加的严格约束,使用应变环状二酰基过氧化物作为牺牲氧化剂对于控制高价钯中间体的反应性及其对多个竞争性还原消除事件的选择性至关重要。机理研究表明该反应通过独特的Pd II /Pd IV 催化途径进行,并且转换限制步骤是底物与钯催化剂的配位。此外,光物理研究表明,不同的N-保护基团和增加的共轭体系对咔唑衍生物的光物理性质有明显的影响。六个绿色指标的计算证明了该催化系统的潜在绿色性。






































 京公网安备 11010802027423号
京公网安备 11010802027423号