当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Visible-light-mediated synthesis of polysubstituted pyrroles via CAr–I reduction triggered 1,5-hydrogen atom transfer process
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-25 , DOI: 10.1039/d4qo00953c Junlei Wang , Qinglin Xie , Guocheng Gao , Guofen Wei , Xinxin Wei , Xuemei Chen , Daohai Zhang , Hongqing Li , Binbin Huang
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-25 , DOI: 10.1039/d4qo00953c Junlei Wang , Qinglin Xie , Guocheng Gao , Guofen Wei , Xinxin Wei , Xuemei Chen , Daohai Zhang , Hongqing Li , Binbin Huang
|
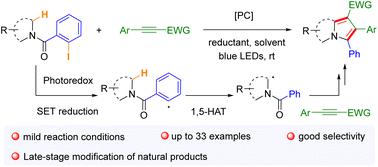
A novel strategy for the construction of polysubstituted pyrroles has been developed through the visible-light-induced single-electron reduction of CAr–I bonds and the following 1,5-hydrogen atom transfer (HAT) process. This protocol facilitates smooth reactions between cyclic 2-iodobenzoyl amides and internal alkynes bearing electron-withdrawing groups (EWGs), affording a wide range of pyrrole skeletons with diverse substitution patterns. Preliminary mechanistic explorations indicate that the 1,5-HAT process triggered by CAr–I activation is crucial to the successful transformation. Scale-up reactions and late-stage modification of natural products further highlight the synthetic potential of this method.
中文翻译:

可见光介导通过 CAr-I 还原触发 1,5-氢原子转移过程合成多取代吡咯
通过可见光诱导的 C Ar -I 键的单电子还原和随后的 1,5-氢原子转移 (HAT) 过程,开发了一种构建多取代吡咯的新策略。该方案促进环状 2-碘苯甲酰酰胺和带有吸电子基团 (EWG) 的内部炔烃之间的顺利反应,提供各种具有不同取代模式的吡咯骨架。初步的机制探索表明,C Ar –I 激活引发的 1,5-HAT 过程对于成功转化至关重要。天然产物的放大反应和后期修饰进一步凸显了该方法的合成潜力。
更新日期:2024-06-25
中文翻译:

可见光介导通过 CAr-I 还原触发 1,5-氢原子转移过程合成多取代吡咯
通过可见光诱导的 C Ar -I 键的单电子还原和随后的 1,5-氢原子转移 (HAT) 过程,开发了一种构建多取代吡咯的新策略。该方案促进环状 2-碘苯甲酰酰胺和带有吸电子基团 (EWG) 的内部炔烃之间的顺利反应,提供各种具有不同取代模式的吡咯骨架。初步的机制探索表明,C Ar –I 激活引发的 1,5-HAT 过程对于成功转化至关重要。天然产物的放大反应和后期修饰进一步凸显了该方法的合成潜力。






































 京公网安备 11010802027423号
京公网安备 11010802027423号