当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Atroposelective Synthesis of C−N Axially Chiral Aminophosphines via Dynamic Kinetic Resolution
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-06-25 , DOI: 10.1002/anie.202409524
Carlos Rodríguez-Franco 1 , Esther Roldán-Molina 1 , Alberto Aguirre-Medina 2 , Rosario Fernández 2 , Valentín Hornillos 1, 2 , José M Lassaletta 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-06-25 , DOI: 10.1002/anie.202409524
Carlos Rodríguez-Franco 1 , Esther Roldán-Molina 1 , Alberto Aguirre-Medina 2 , Rosario Fernández 2 , Valentín Hornillos 1, 2 , José M Lassaletta 1
Affiliation
|
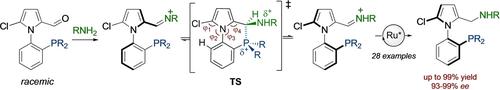
Axially chiral P,N ligands were synthesized through ruthenium-catalyzed ATH of in situ generated iminophosphines. The transient interaction between iminium and phosphine groups as a Lewis acid-base pair causes the labilization of the stereogenic axis, enabling the scenario for a DKR. The reaction proceeds with good to high yields and reaching up to 99 % ee across a range of substrates, including both dicyclohexyl- and diphenylphosphino derivatives.
中文翻译:

通过动态动力学拆分催化旋转选择性合成 C−N 轴向手性氨基膦
通过钌催化原位生成亚氨基膦的ATH合成轴向手性P 、 N配体。亚胺和膦基团之间作为路易斯酸碱对的瞬时相互作用导致立体轴不稳定,从而实现了 DKR 的情况。该反应以良好到高的产率进行,在一系列底物(包括二环己基膦衍生物和二苯基膦衍生物)中,产率高达 99% ee 。
更新日期:2024-06-25
中文翻译:

通过动态动力学拆分催化旋转选择性合成 C−N 轴向手性氨基膦
通过钌催化原位生成亚氨基膦的ATH合成轴向手性P 、 N配体。亚胺和膦基团之间作为路易斯酸碱对的瞬时相互作用导致立体轴不稳定,从而实现了 DKR 的情况。该反应以良好到高的产率进行,在一系列底物(包括二环己基膦衍生物和二苯基膦衍生物)中,产率高达 99% ee 。

































 京公网安备 11010802027423号
京公网安备 11010802027423号