当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unveiling the Potential of Ca/Cu Composite Sorbents for CO2 Capture: A Precursor Perspective
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-06-25 , DOI: 10.1021/acs.iecr.4c01037 Yanbin Hu 1 , Xilei Liu 1 , Yong Li 1 , Yuxin Jiang 1 , Yuxin Ma 1 , Jian Chen 1 , Yuanchao Xue 1 , Mengru Wang 1 , Youshi Li 1 , Mingdi Li 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-06-25 , DOI: 10.1021/acs.iecr.4c01037 Yanbin Hu 1 , Xilei Liu 1 , Yong Li 1 , Yuxin Jiang 1 , Yuxin Ma 1 , Jian Chen 1 , Yuanchao Xue 1 , Mengru Wang 1 , Youshi Li 1 , Mingdi Li 1
Affiliation
|
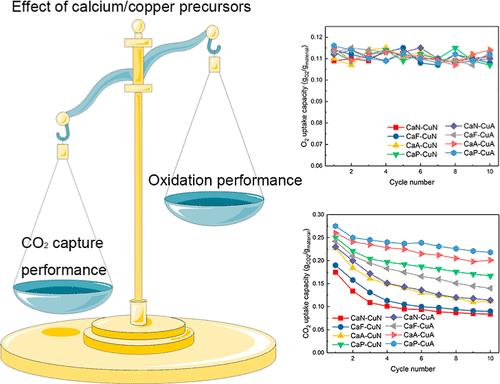
The combined Ca/Cu process holds promise as a CO2 capture technique utilizing chemical looping combustion to provide heat for regenerating CaO-based sorbents in a calcium looping configuration through the Ca/Cu composite sorbents. Developing Ca/Cu composite sorbents with high reactivity is crucial for advancing combined Ca/Cu technology. However, these sorbents encounter a rapid decline in the CO2 capture performance, remaining a significant problem to be addressed. Herein, various calcium/copper precursors, comprising copper acetate, copper nitrate, calcium propionate, calcium acetate, calcium formate, and calcium nitrate, were utilized to synthesize Ca/Cu composite sorbents using a Pechini method. The results reveal that the selection of calcium and copper precursors significantly affected the CO2 capture performance. Utilizing organic salts as calcium and/or copper precursors proved beneficial in enhancing the CO2 capture performance, particularly when employing organic salts with high molecular weights (e.g., copper acetate, calcium propionate, and calcium acetate). The sorbent synthesized using calcium propionate and copper acetate possessed the highest CO2 capture performance, achieving a final CO2 uptake capacity of 0.22 gCO2/gmaterial in the 10th cycle while retaining 80% of its initial reactivity. In contrast, the sorbent synthesized using calcium nitrate and copper nitrate showed the poorest CO2 capture performance, with an initial capacity of 0.18 gCO2/gmaterial and a final capacity of 0.08 gCO2/gmaterial in the 10th cycle. In comparison to the significant impact on the CO2 capture performance, the selection of precursors had a minimal effect on oxidation performance. Regardless of the precursors used, all the sorbents exhibited commendable oxidation performance, achieving oxidation conversions exceeding 90%. Additionally, systematic investigations were conducted on process conditions encompassing the reaction atmosphere and temperature during the oxidation and carbonation stages. The findings indicate that process conditions had a greater impact on the CO2 capture performance than on the oxidation performance.
中文翻译:

揭示 Ca/Cu 复合吸附剂捕集二氧化碳的潜力:前驱体的视角
组合的 Ca/Cu 工艺有望作为一种 CO 2 捕获技术,利用化学循环燃烧为通过 Ca/Cu 复合吸附剂的钙循环配置中的 CaO 基吸附剂的再生提供热量。开发具有高反应活性的钙/铜复合吸附剂对于推进钙/铜组合技术至关重要。然而,这些吸附剂的 CO 2 捕获性能迅速下降,仍然是一个需要解决的重大问题。在此,使用包括乙酸铜、硝酸铜、丙酸钙、乙酸钙、甲酸钙和硝酸钙的各种钙/铜前体,使用Pechini方法合成Ca/Cu复合吸附剂。结果表明,钙和铜前体的选择显着影响CO 2 捕获性能。使用有机盐作为钙和/或铜前体被证明有益于增强CO 2 捕获性能,特别是当使用高分子量有机盐(例如乙酸铜、丙酸钙和乙酸钙)时。使用丙酸钙和乙酸铜合成的吸附剂具有最高的CO 2 捕获性能,最终CO 2 吸收容量为0.22g CO2 /g material 在第 10 个循环中,同时保留其初始反应性的 80%。相比之下,使用硝酸钙和硝酸铜合成的吸附剂表现出最差的CO 2 捕获性能,初始容量为0.18 g CO2 /g material ,第 10 次循环的最终容量为 0.08 g CO2 /g material 。 与对 CO 2 捕获性能的显着影响相比,前体的选择对氧化性能的影响最小。无论使用何种前驱体,所有吸附剂都表现出值得称赞的氧化性能,氧化转化率超过 90%。此外,还对氧化和碳酸化阶段的反应气氛和温度等工艺条件进行了系统研究。研究结果表明,工艺条件对 CO 2 捕获性能的影响大于对氧化性能的影响。
更新日期:2024-06-25
中文翻译:

揭示 Ca/Cu 复合吸附剂捕集二氧化碳的潜力:前驱体的视角
组合的 Ca/Cu 工艺有望作为一种 CO 2 捕获技术,利用化学循环燃烧为通过 Ca/Cu 复合吸附剂的钙循环配置中的 CaO 基吸附剂的再生提供热量。开发具有高反应活性的钙/铜复合吸附剂对于推进钙/铜组合技术至关重要。然而,这些吸附剂的 CO 2 捕获性能迅速下降,仍然是一个需要解决的重大问题。在此,使用包括乙酸铜、硝酸铜、丙酸钙、乙酸钙、甲酸钙和硝酸钙的各种钙/铜前体,使用Pechini方法合成Ca/Cu复合吸附剂。结果表明,钙和铜前体的选择显着影响CO 2 捕获性能。使用有机盐作为钙和/或铜前体被证明有益于增强CO 2 捕获性能,特别是当使用高分子量有机盐(例如乙酸铜、丙酸钙和乙酸钙)时。使用丙酸钙和乙酸铜合成的吸附剂具有最高的CO 2 捕获性能,最终CO 2 吸收容量为0.22g CO2 /g material 在第 10 个循环中,同时保留其初始反应性的 80%。相比之下,使用硝酸钙和硝酸铜合成的吸附剂表现出最差的CO 2 捕获性能,初始容量为0.18 g CO2 /g material ,第 10 次循环的最终容量为 0.08 g CO2 /g material 。 与对 CO 2 捕获性能的显着影响相比,前体的选择对氧化性能的影响最小。无论使用何种前驱体,所有吸附剂都表现出值得称赞的氧化性能,氧化转化率超过 90%。此外,还对氧化和碳酸化阶段的反应气氛和温度等工艺条件进行了系统研究。研究结果表明,工艺条件对 CO 2 捕获性能的影响大于对氧化性能的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号