当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh(III)-Catalyzed Selective C−H Alkylation and Alkenylation of 1,2,3-Benzotriazinones with Maleimides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-06-24 , DOI: 10.1002/adsc.202400410 Shang-Shi Zhang 1 , Lin Xiao 1 , Dan-Ting Shen 1 , Wen-Xuan Zou 1 , Jia-Lin Song 1 , Jiaohang Wei 1 , Xiang Liu 1 , Luyong Zhang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-06-24 , DOI: 10.1002/adsc.202400410 Shang-Shi Zhang 1 , Lin Xiao 1 , Dan-Ting Shen 1 , Wen-Xuan Zou 1 , Jia-Lin Song 1 , Jiaohang Wei 1 , Xiang Liu 1 , Luyong Zhang 1
Affiliation
|
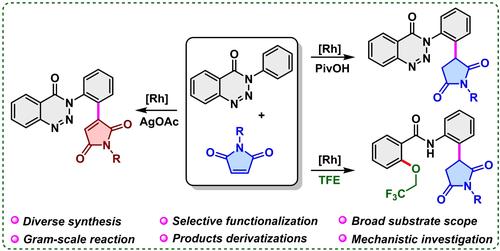
Herein, we describe a condition‐based switchable Rh(III)‐catalyzed C‐H alkylation and alkenylation of 1,2,3‐benzotriazinones with maleimides, where triazinone serves as a directing group rather than its traditional role denitrogenative precursor. This strategy enables the selective synthesis of diverse 3‐arylated succinimides and 3‐arylated maleimides in up to 99% yield with broad substrate scope (48 examples). Furthermore, for the first time, a tandem C‐H alkylation and denitrogenative coupling of 1,2,3‐benzotriazinones has been realized by slightly modifying the reaction conditions. Additionally, gram‐scale reactions and product derivatizations were conducted to demonstrate the synthetic utility.
中文翻译:

Rh(III)-催化 1,2,3-苯并三嗪酮与马来酰亚胺的选择性 C−H 烷基化和烯基化
在此,我们描述了基于条件的可切换Rh(III)催化的1,2,3-苯并三嗪酮与马来酰亚胺的C-H烷基化和烯基化,其中三嗪酮充当导向基团而不是其传统的脱氮前体作用。该策略能够选择性合成多种 3-芳基化琥珀酰亚胺和 3-芳基化马来酰亚胺,产率高达 99%,底物范围广泛(48 个例子)。此外,通过稍微修改反应条件,首次实现了1,2,3-苯并三嗪酮的串联C-H烷基化和脱氮偶联。此外,还进行了克级反应和产物衍生化来证明合成效用。
更新日期:2024-06-24
中文翻译:

Rh(III)-催化 1,2,3-苯并三嗪酮与马来酰亚胺的选择性 C−H 烷基化和烯基化
在此,我们描述了基于条件的可切换Rh(III)催化的1,2,3-苯并三嗪酮与马来酰亚胺的C-H烷基化和烯基化,其中三嗪酮充当导向基团而不是其传统的脱氮前体作用。该策略能够选择性合成多种 3-芳基化琥珀酰亚胺和 3-芳基化马来酰亚胺,产率高达 99%,底物范围广泛(48 个例子)。此外,通过稍微修改反应条件,首次实现了1,2,3-苯并三嗪酮的串联C-H烷基化和脱氮偶联。此外,还进行了克级反应和产物衍生化来证明合成效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号