当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fabrication, structural elucidation, and DFT calculation of some new hydrophilic metal chelates based on N N′-(1-methyl-2-oxoindolin-3-ylidene)benzohydrazide ligand: Pharmaceutical studies and molecular docking approach
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2024-06-23 , DOI: 10.1002/aoc.7593 Ahmed M. Abu‐Dief 1, 2 , Omran A. Omran 1 , Mehran Feizi‐Dehnayebi 3 , Abdulmajeed Alqurashi 4 , Inam Omar 2 , Dalal Alhashmialameer 5 , Ahmad Desoky M. Mohamad 1
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2024-06-23 , DOI: 10.1002/aoc.7593 Ahmed M. Abu‐Dief 1, 2 , Omran A. Omran 1 , Mehran Feizi‐Dehnayebi 3 , Abdulmajeed Alqurashi 4 , Inam Omar 2 , Dalal Alhashmialameer 5 , Ahmad Desoky M. Mohamad 1
Affiliation
|
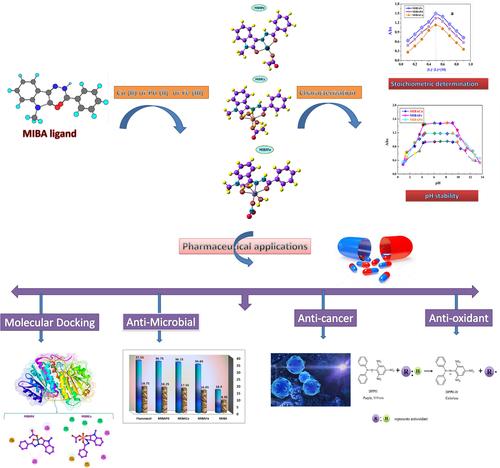
Some novel FeIII, CuII, and PdII chelates incorporating N′-(1-methyl-2-oxoindolin-3-ylidene)benzohydrazide (MIBA) were fabricated. The tested compounds were investigated using thermogravimetric analysis (TGA), CHN, spectra analysis (IR, mass spectra, and NMR), melting point, magnetic moments, molar conductance, ultraviolet–visible spectroscopy, powder X-ray diffraction, and computational studies. The conductance results showed that the tested FeIII, CuII, and PdII chelates are electrolytes. Magnetic and electronic spectra are applied to deduce the coordinating ability of the tested ligand, and the geometric structure of the studied chelates is found to be octahedral, distorted octahedral, and square planar for FeIII, CuII, and PdII chelates, respectively. The TGA study of these studied complexes displays that the hydrated H2O molecules, acetate, and nitrate are removed in the first and second degradation steps followed directly by degradation of the studied ligand leaving metal oxide as residue. The thermodynamic factors, like ΔS*, ΔH*, E*, A, and ΔG* are evaluated from the TGA curves and explained. The density functional theory (DFT)/B3LYP computation method was applied for the estimation of the molecular electrostatic potential (MEP; highest occupied molecular orbital [HOMO] and lowest unoccupied molecular orbital [LUMO]) energy for the studied compounds. In an in vitro study, the antimicrobial effects of the prepared compounds were screened on various strains of bacteria and fungi. It was found that tested compounds exposed a good biological efficacy through IC50 results close to reference drugs and antitumor potential against (MCF-7, Hep-G2, and HC-T116) cell lines. The data obtained displayed that the studied chelates showed promising antitumor activity. The studied metal chelates were screened for in vitro antioxidant efficacy using DPPH assay. The studied compounds explained dynamic satisfying performance. Also, the crystal structures of breast cancer protein (PDB ID: 3HB5) and Escherichia coli (PDB ID: 2VF5) were performed by molecular docking simulation. Data of docking simulation suggestions are which tested compounds have biological behavior as well as have obvious benefit in the pharmaceutical business.
中文翻译:

基于NN′-(1-甲基-2-氧吲哚啉-3-亚基)苯甲酰肼配体的一些新型亲水性金属螯合物的制备、结构解析和DFT计算:药物研究和分子对接方法
制备了一些包含 N'-(1-甲基-2-氧代吲哚啉-3-亚基)苯甲酰肼 (MIBA) 的新型 Fe III 、Cu II和 Pd II螯合物。使用热重分析 (TGA)、CHN、光谱分析(IR、质谱和 NMR)、熔点、磁矩、摩尔电导、紫外-可见光谱、粉末 X 射线衍射和计算研究对测试化合物进行了研究。电导结果表明,所测试的Fe III 、Cu II和Pd II螯合物都是电解质。应用磁谱和电子谱推导了测试配体的配位能力,发现所研究的螯合物的几何结构分别为八面体、扭曲八面体和方形平面(Fe III 、Cu II和 Pd II螯合物)。对这些所研究的配合物的 TGA 研究表明,水合 H 2 O 分子、乙酸盐和硝酸盐在第一和第二降解步骤中被去除,随后直接降解所研究的配体,留下金属氧化物作为残留物。根据 TGA 曲线评估并解释了热力学因素,如 ΔS*、ΔH*、E*、A 和 ΔG*。应用密度泛函理论 (DFT)/B3LYP 计算方法来估计所研究化合物的分子静电势(MEP;最高占据分子轨道 [HOMO] 和最低未占据分子轨道 [LUMO])能量。在体外研究中,筛选了所制备的化合物对各种细菌和真菌菌株的抗菌作用。 结果发现,测试化合物具有与参考药物接近的 IC 50结果和针对(MCF-7、Hep-G2 和 HC-T116)细胞系的抗肿瘤潜力的良好生物功效。获得的数据表明,所研究的螯合物显示出有希望的抗肿瘤活性。使用 DPPH 测定筛选所研究的金属螯合物的体外抗氧化功效。研究的化合物解释了动态令人满意的性能。此外,还通过分子对接模拟对乳腺癌蛋白(PDB ID:3HB5)和大肠杆菌(PDB ID:2VF5)的晶体结构进行了分析。对接模拟建议的数据是哪些受测化合物具有生物学行为,并且对制药业务具有明显的效益。
更新日期:2024-06-23
中文翻译:

基于NN′-(1-甲基-2-氧吲哚啉-3-亚基)苯甲酰肼配体的一些新型亲水性金属螯合物的制备、结构解析和DFT计算:药物研究和分子对接方法
制备了一些包含 N'-(1-甲基-2-氧代吲哚啉-3-亚基)苯甲酰肼 (MIBA) 的新型 Fe III 、Cu II和 Pd II螯合物。使用热重分析 (TGA)、CHN、光谱分析(IR、质谱和 NMR)、熔点、磁矩、摩尔电导、紫外-可见光谱、粉末 X 射线衍射和计算研究对测试化合物进行了研究。电导结果表明,所测试的Fe III 、Cu II和Pd II螯合物都是电解质。应用磁谱和电子谱推导了测试配体的配位能力,发现所研究的螯合物的几何结构分别为八面体、扭曲八面体和方形平面(Fe III 、Cu II和 Pd II螯合物)。对这些所研究的配合物的 TGA 研究表明,水合 H 2 O 分子、乙酸盐和硝酸盐在第一和第二降解步骤中被去除,随后直接降解所研究的配体,留下金属氧化物作为残留物。根据 TGA 曲线评估并解释了热力学因素,如 ΔS*、ΔH*、E*、A 和 ΔG*。应用密度泛函理论 (DFT)/B3LYP 计算方法来估计所研究化合物的分子静电势(MEP;最高占据分子轨道 [HOMO] 和最低未占据分子轨道 [LUMO])能量。在体外研究中,筛选了所制备的化合物对各种细菌和真菌菌株的抗菌作用。 结果发现,测试化合物具有与参考药物接近的 IC 50结果和针对(MCF-7、Hep-G2 和 HC-T116)细胞系的抗肿瘤潜力的良好生物功效。获得的数据表明,所研究的螯合物显示出有希望的抗肿瘤活性。使用 DPPH 测定筛选所研究的金属螯合物的体外抗氧化功效。研究的化合物解释了动态令人满意的性能。此外,还通过分子对接模拟对乳腺癌蛋白(PDB ID:3HB5)和大肠杆菌(PDB ID:2VF5)的晶体结构进行了分析。对接模拟建议的数据是哪些受测化合物具有生物学行为,并且对制药业务具有明显的效益。







































 京公网安备 11010802027423号
京公网安备 11010802027423号