当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of Novel (+)-Nootkatone-Based Amine Derivatives as Potential Insecticide Candidates
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-06-24 , DOI: 10.1021/acs.jafc.4c02697 Yong Guo 1, 2 , Meiyue Han 2 , Yan Zhong 1 , Xueyu Li 2 , Songlin Hu 1 , Ruige Yang 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-06-24 , DOI: 10.1021/acs.jafc.4c02697 Yong Guo 1, 2 , Meiyue Han 2 , Yan Zhong 1 , Xueyu Li 2 , Songlin Hu 1 , Ruige Yang 1, 2
Affiliation
|
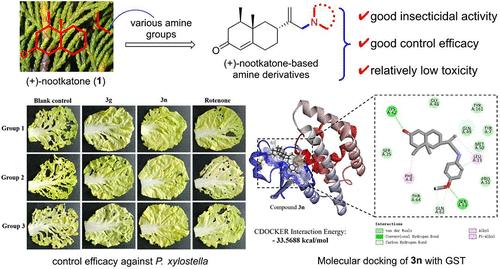
To discover novel natural product-based insecticides, a series of (+)-nootkatone-based amine derivatives 3a–t were prepared and evaluated for their insecticidal activities against Mythimna separata Walker, Myzus persicae Sulzer, and Plutella xylostella Linnaeus. Insecticidal assays showed that most of the title (+)-nootkatone derivatives exhibited stronger insecticidal activities against three insect pests than the precursor (+)-nootkatone after the introduction of amine groups on the parent (+)-nootkatone. Compounds 3a, 3d, 3h, 3m, 3n, 3p, and 3r displayed more promising growth inhibitory (GI) effect against M. separata than the commercially available botanical insecticide toosendanin. Compound 3o exhibited the most potent aphicidal activity with an LD50 value of 0.011 μg/larvae, which was 2.09-fold higher than the positive control rotenone. Additionally, compounds 3g and 3n showed more promising larvicidal activity against P. xylostella with LC50 values of 260 and 230 mg/L, respectively, superior to that of rotenone (460 mg/L). Moreover, derivatives 3g and 3n exhibited better control efficacy toward P. xylostella than rotenone under greenhouse conditions. Preliminary mechanistic studies revealed that derivative 3n could inhibit the activity of glutathione S-transferase (GST) in P. xylostella and thus exerted larvicidal activity, and molecular docking further demonstrated that 3n could interact well with some amino acid residues of GST. Finally, the toxicity assay suggested that derivatives 3g and 3n were relatively less toxic to nontarget organisms. These findings will provide insights into the development of (+)-nootkatone derivatives as green pesticides.
中文翻译:

发现新型 (+)-Nootkatone 胺衍生物作为潜在杀虫剂候选物
为了发现新型天然产物杀虫剂,制备了一系列基于 (+)-nootkatone 的胺衍生物 3a-t,并评估了它们对粘虫、桃蚜和小菜蛾的杀虫活性。杀虫试验表明,在母体(+)-诺特卡酮上引入胺基后,大多数标题(+)-诺特卡酮衍生物对三种害虫表现出比前体(+)-诺特卡酮更强的杀虫活性。化合物3a、3d、3h、3m、3n、3p和3r对M. separata表现出比市售植物杀虫剂川楝素更有希望的生长抑制(GI)作用。化合物3o表现出最强的杀蚜活性,LD 50 值为0.011 μg/幼虫,比阳性对照鱼藤酮高2.09倍。此外,化合物3g和3n对小菜蛾表现出更有希望的杀幼虫活性,LC 50 值分别为260和230 mg/L,优于鱼藤酮(460 mg/L)。此外,在温室条件下,衍生物3g和3n对小菜蛾的防治效果优于鱼藤酮。初步机理研究表明,3n衍生物可以抑制小菜蛾体内谷胱甘肽S-转移酶(GST)的活性,从而发挥杀幼虫活性,分子对接进一步证明3n可以与GST的部分氨基酸残基有良好的相互作用。最后,毒性测定表明衍生物3g和3n对非目标生物的毒性相对较小。这些发现将为 (+)-nootkatone 衍生物作为绿色农药的开发提供见解。
更新日期:2024-06-24
中文翻译:

发现新型 (+)-Nootkatone 胺衍生物作为潜在杀虫剂候选物
为了发现新型天然产物杀虫剂,制备了一系列基于 (+)-nootkatone 的胺衍生物 3a-t,并评估了它们对粘虫、桃蚜和小菜蛾的杀虫活性。杀虫试验表明,在母体(+)-诺特卡酮上引入胺基后,大多数标题(+)-诺特卡酮衍生物对三种害虫表现出比前体(+)-诺特卡酮更强的杀虫活性。化合物3a、3d、3h、3m、3n、3p和3r对M. separata表现出比市售植物杀虫剂川楝素更有希望的生长抑制(GI)作用。化合物3o表现出最强的杀蚜活性,LD 50 值为0.011 μg/幼虫,比阳性对照鱼藤酮高2.09倍。此外,化合物3g和3n对小菜蛾表现出更有希望的杀幼虫活性,LC 50 值分别为260和230 mg/L,优于鱼藤酮(460 mg/L)。此外,在温室条件下,衍生物3g和3n对小菜蛾的防治效果优于鱼藤酮。初步机理研究表明,3n衍生物可以抑制小菜蛾体内谷胱甘肽S-转移酶(GST)的活性,从而发挥杀幼虫活性,分子对接进一步证明3n可以与GST的部分氨基酸残基有良好的相互作用。最后,毒性测定表明衍生物3g和3n对非目标生物的毒性相对较小。这些发现将为 (+)-nootkatone 衍生物作为绿色农药的开发提供见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号