Cell ( IF 45.5 ) Pub Date : 2024-06-24 , DOI: 10.1016/j.cell.2024.05.039 Sara R. Savage , Xinpei Yi , Jonathan T. Lei , Bo Wen , Hongwei Zhao , Yuxing Liao , Eric J. Jaehnig , Lauren K. Somes , Paul W. Shafer , Tobie D. Lee , Zile Fu , Yongchao Dou , Zhiao Shi , Daming Gao , Valentina Hoyos , Qiang Gao , Bing Zhang
|
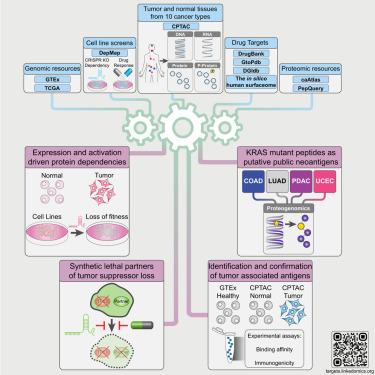
Fewer than 200 proteins are targeted by cancer drugs approved by the Food and Drug Administration (FDA). We integrate Clinical Proteomic Tumor Analysis Consortium (CPTAC) proteogenomics data from 1,043 patients across 10 cancer types with additional public datasets to identify potential therapeutic targets. Pan-cancer analysis of 2,863 druggable proteins reveals a wide abundance range and identifies biological factors that affect mRNA-protein correlation. Integration of proteomic data from tumors and genetic screen data from cell lines identifies protein overexpression- or hyperactivation-driven druggable dependencies, enabling accurate predictions of effective drug targets. Proteogenomic identification of synthetic lethality provides a strategy to target tumor suppressor gene loss. Combining proteogenomic analysis and MHC binding prediction prioritizes mutant KRAS peptides as promising public neoantigens. Computational identification of shared tumor-associated antigens followed by experimental confirmation nominates peptides as immunotherapy targets. These analyses, summarized at https://targets.linkedomics.org, form a comprehensive landscape of protein and peptide targets for companion diagnostics, drug repurposing, and therapy development.
中文翻译:

泛癌蛋白质基因组学扩大了治疗靶点的范围
美国食品和药物管理局 (FDA) 批准的抗癌药物靶向的蛋白质不到 200 种。我们将临床蛋白质组肿瘤分析联盟 (CPTAC) 1043 名患者的 10 种癌症类型的蛋白质基因组数据与其他公共数据集相整合,以确定潜在的治疗靶点。对 2,863 种可药物蛋白的泛癌分析揭示了广泛的丰度范围,并确定了影响 mRNA-蛋白相关性的生物因素。肿瘤蛋白质组数据和细胞系遗传筛选数据的整合可识别蛋白质过度表达或过度激活驱动的药物依赖性,从而能够准确预测有效药物靶点。合成致死性的蛋白质组学鉴定提供了一种针对肿瘤抑制基因丢失的策略。结合蛋白质组分析和 MHC 结合预测,优先考虑突变 KRAS 肽作为有前途的公共新抗原。通过计算鉴定共享的肿瘤相关抗原,然后通过实验确认,指定肽作为免疫治疗靶点。这些分析总结于 https://targets.linkedomics.org,形成了用于伴随诊断、药物再利用和治疗开发的蛋白质和肽靶标的全面景观。











































 京公网安备 11010802027423号
京公网安备 11010802027423号