Cell ( IF 45.5 ) Pub Date : 2024-06-24 , DOI: 10.1016/j.cell.2024.05.051 Madeleine Linneberg-Agerholm , Annika Charlotte Sell , Alba Redó-Riveiro , Marta Perera , Martin Proks , Teresa E. Knudsen , Antonio Barral , Miguel Manzanares , Joshua M. Brickman
|
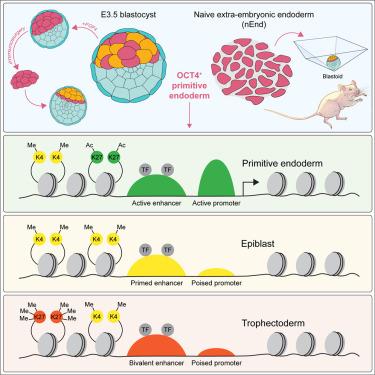
Mammalian blastocyst formation involves the specification of the trophectoderm followed by the differentiation of the inner cell mass into embryonic epiblast and extra-embryonic primitive endoderm (PrE). During this time, the embryo maintains a window of plasticity and can redirect its cellular fate when challenged experimentally. In this context, we found that the PrE alone was sufficient to regenerate a complete blastocyst and continue post-implantation development. We identify an in vitro population similar to the early PrE in vivo that exhibits the same embryonic and extra-embryonic potency and can form complete stem cell-based embryo models, termed blastoids. Commitment in the PrE is suppressed by JAK/STAT signaling, collaborating with OCT4 and the sustained expression of a subset of pluripotency-related transcription factors that safeguard an enhancer landscape permissive for multi-lineage differentiation. Our observations support the notion that transcription factor persistence underlies plasticity in regulative development and highlight the importance of the PrE in perturbed development.
中文翻译:

原始内胚层支持谱系可塑性以实现调节性发育
哺乳动物囊胚的形成涉及滋养外胚层的特化,随后内细胞团分化为胚胎外胚层和胚胎外原始内胚层(PrE)。在此期间,胚胎保持着可塑性窗口,并且在受到实验挑战时可以改变其细胞命运。在这种情况下,我们发现仅 PrE 就足以再生完整的囊胚并继续植入后发育。我们鉴定出与体内早期 PrE 类似的体外群体,其表现出相同的胚胎和胚胎外效力,并且可以形成完整的基于干细胞的胚胎模型,称为胚泡。 PrE 中的承诺受到 JAK/STAT 信号传导的抑制,与 OCT4 和多能性相关转录因子子集的持续表达相配合,保护了允许多谱系分化的增强子景观。我们的观察结果支持这样的观点,即转录因子的持久性是调节发育中可塑性的基础,并强调了 PrE 在扰动发育中的重要性。






































 京公网安备 11010802027423号
京公网安备 11010802027423号