当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the influence of metal cations on individual hydrogen bonds in Watson–Crick guanine–cytosine DNA base pair: An interacting quantum atoms analysis
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-06-24 , DOI: 10.1002/jcc.27441 F Pakzad 1 , K Eskandari 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-06-24 , DOI: 10.1002/jcc.27441 F Pakzad 1 , K Eskandari 1
Affiliation
|
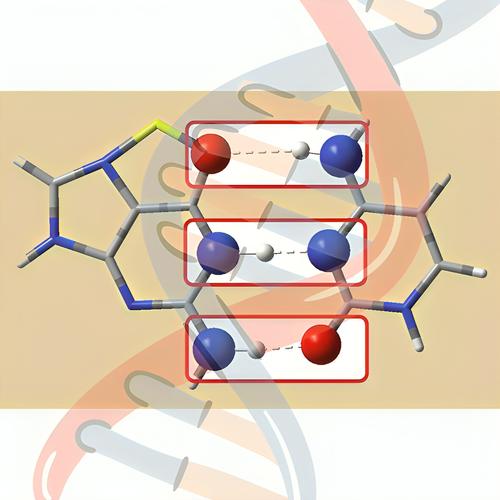
This study delves into the nature of individual hydrogen bonds and the relationship between metal cations and hydrogen bonding in the Watson–Crick guanine–cytosine (GC) base pair and its alkali and alkaline earth cation-containing complexes (Mn+–GC). The findings reveal how metal cations affect the nature and strength of individual hydrogen bonds. The study employs interacting quantum atoms (IQA) analysis to comprehensively understand three individual hydrogen bonds within the GC base pair and its cationic derivatives. These analyses unveil the nature and strength of hydrogen bonds and serve as a valuable reference for exploring the impact of cations (and other factors) on each hydrogen bond. All the H
中文翻译:

探索金属阳离子对 Watson-Crick 鸟嘌呤-胞嘧啶 DNA 碱基对中单个氢键的影响:相互作用的量子原子分析
这项研究深入研究了沃森-克里克鸟嘌呤-胞嘧啶(GC)碱基对及其含碱金属和碱土金属阳离子的配合物(M n+ -GC)中单个氢键的性质以及金属阳离子和氢键之间的关系。研究结果揭示了金属阳离子如何影响单个氢键的性质和强度。该研究采用相互作用量子原子 (IQA) 分析来全面了解 GC 碱基对及其阳离子衍生物内的三个单独的氢键。这些分析揭示了氢键的性质和强度,并为探索阳离子(和其他因素)对每个氢键的影响提供了有价值的参考。所有的H ⋯ ⋯
更新日期:2024-06-24
中文翻译:

探索金属阳离子对 Watson-Crick 鸟嘌呤-胞嘧啶 DNA 碱基对中单个氢键的影响:相互作用的量子原子分析
这项研究深入研究了沃森-克里克鸟嘌呤-胞嘧啶(GC)碱基对及其含碱金属和碱土金属阳离子的配合物(M n+ -GC)中单个氢键的性质以及金属阳离子和氢键之间的关系。研究结果揭示了金属阳离子如何影响单个氢键的性质和强度。该研究采用相互作用量子原子 (IQA) 分析来全面了解 GC 碱基对及其阳离子衍生物内的三个单独的氢键。这些分析揭示了氢键的性质和强度,并为探索阳离子(和其他因素)对每个氢键的影响提供了有价值的参考。所有的H































 京公网安备 11010802027423号
京公网安备 11010802027423号