当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereo-selective synthesis of complex dienes and eneynes by sequential hydroarylation and olefinic C–H functionalization
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-24 , DOI: 10.1039/d4qo00726c Yuhang Zhu 1 , Xiaoli Li 1 , Cheng Zhang 1 , Xiuying Liu 1 , Linzhi Huang 1 , Yongbo Zhang 2 , Chao Shen 3 , Liyuan Ding 1 , Guofu Zhong 1, 4 , Jian Zhang 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-24 , DOI: 10.1039/d4qo00726c Yuhang Zhu 1 , Xiaoli Li 1 , Cheng Zhang 1 , Xiuying Liu 1 , Linzhi Huang 1 , Yongbo Zhang 2 , Chao Shen 3 , Liyuan Ding 1 , Guofu Zhong 1, 4 , Jian Zhang 1
Affiliation
|
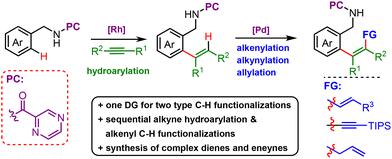
We present a stereo-selective preparation of multi-substituted dienes and eneynes by sequential hydroarylation of internal alkynes via five-membered rhodacycle and olefinic C–H functionalization, including alkenylation, alkynylation and allylation through seven-membered endo-cyclopalladation, both enabled by the N,N-bidentate chelation assistance of pyrazinamide or picolinamide. The robustness and generality of the protocol have been demonstrated by the smooth conversion of a wide range of benzyl amides to afford up to 92% yields with E/Z ratio selectivity up to >99/1, as well as a successful gram-scaled synthesis. Further chemical derivations and photophysical properties of products were also examined.
中文翻译:

通过顺序加氢芳基化和烯属 C-H 官能化立体选择性合成复杂二烯和烯炔
我们提出了一种多取代二烯和烯炔的立体选择性制备方法,通过五元铑环和烯属 C-H 官能化对内部炔烃进行连续氢芳基化,包括通过七元内环钯化进行烯基化、炔基化和烯基化,这两种方法均由吡嗪酰胺或吡啶甲酰胺的 N,N-二齿螯合辅助。该方案的稳健性和通用性已通过多种苄基酰胺的顺利转化得到证明,产率高达 92%,E/Z 比率选择性高达 >99/1,以及成功的克级合成。还检查了产品的进一步化学衍生和光物理性质。
更新日期:2024-06-24
中文翻译:

通过顺序加氢芳基化和烯属 C-H 官能化立体选择性合成复杂二烯和烯炔
我们提出了一种多取代二烯和烯炔的立体选择性制备方法,通过五元铑环和烯属 C-H 官能化对内部炔烃进行连续氢芳基化,包括通过七元内环钯化进行烯基化、炔基化和烯基化,这两种方法均由吡嗪酰胺或吡啶甲酰胺的 N,N-二齿螯合辅助。该方案的稳健性和通用性已通过多种苄基酰胺的顺利转化得到证明,产率高达 92%,E/Z 比率选择性高达 >99/1,以及成功的克级合成。还检查了产品的进一步化学衍生和光物理性质。






































 京公网安备 11010802027423号
京公网安备 11010802027423号