当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solvent Exchange Dynamics in M2(dobdc): An Interplay among Binding Strength, Exchange Kinetics, and Cooperativity
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-21 , DOI: 10.1021/jacs.4c05355 Hochul Woo 1, 2 , John W. Robinson 1 , Adam J. Matzger 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-21 , DOI: 10.1021/jacs.4c05355 Hochul Woo 1, 2 , John W. Robinson 1 , Adam J. Matzger 1, 2
Affiliation
|
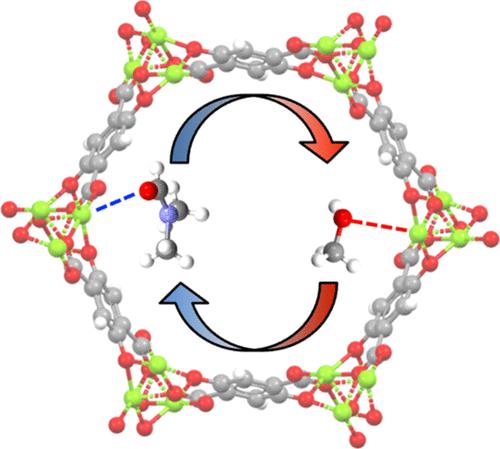
Solvent exchange is a crucial step in ensuring the complete activation of metal–organic frameworks (MOFs); however, the conditions for solvent exchange vary among MOFs, even within the isostructural variants. This study examines the factors contributing to solvent exchange by investigating the isostructural M2(dobdc) (M═Mg, Co, Zn) series. Common solvents N,N-dimethylformamide (DMF), ethanol (EtOH), and methanol (MeOH) are employed to assess the solvent exchange at coordinatively unsaturated sites (CUS) of M2(dobdc). By monitoring both solvents released from the MOF during solvent exchange and the coordination environment of metals within the MOF, a picture is constructed of exchange rates during early stages of solvent exchange as well as expulsion of the last traces of bound solvents. This differentiation is achieved by a combination of bulk monitoring of solvent phase composition and microscopic application of Raman spectroscopy on the single-crystal level. The kinetics of solvent replacement is revealed to have a substantial contribution from cooperativity; this phenomenon is observed in both the forward and reverse directions. Thermogravimetric analysis coupled with IR spectroscopy and density functional theory (DFT) calculations are employed to elucidate the relationship between solvent exchange rates and solvent binding energy. The solvent exchange rates are determined by the kinetic barriers of solvent exchange that do not follow the order of the solvent binding affinity. This work contributes to understanding the solvent exchange of MOFs by examining the interplay among the binding strength, exchange kinetics, and cooperativity. It further provides valuable insights for scrutinizing MOF activation protocols.
中文翻译:

M2(dobdc) 中的溶剂交换动力学:结合强度、交换动力学和协同性之间的相互作用
溶剂交换是确保金属有机框架(MOF)完全活化的关键步骤;然而,MOF 之间的溶剂交换条件各不相同,甚至在同构变体中也是如此。本研究通过研究同构 M 2 (dobdc) (M=Mg, Co, Zn) 系列来研究影响溶剂交换的因素。使用常用溶剂 N,N-二甲基甲酰胺 (DMF)、乙醇 (EtOH) 和甲醇 (MeOH) 来评估 M 2 (dobdc) 配位不饱和位点 (CUS) 的溶剂交换。通过监测溶剂交换过程中 MOF 释放的溶剂以及 MOF 内金属的配位环境,可以构建出溶剂交换早期阶段的交换率以及最后痕量结合溶剂的排出情况。这种区分是通过溶剂相成分的整体监测和单晶水平上拉曼光谱的显微应用相结合来实现的。研究表明,溶剂置换的动力学对协同性有很大贡献;这种现象在正向和反向都可以观察到。热重分析与红外光谱和密度泛函理论 (DFT) 计算相结合,用于阐明溶剂交换速率和溶剂结合能之间的关系。溶剂交换速率由溶剂交换的动力学势垒决定,不遵循溶剂结合亲和力的顺序。这项工作通过研究结合强度、交换动力学和协同性之间的相互作用,有助于理解 MOF 的溶剂交换。它进一步为审查 MOF 激活协议提供了宝贵的见解。
更新日期:2024-06-21
中文翻译:

M2(dobdc) 中的溶剂交换动力学:结合强度、交换动力学和协同性之间的相互作用
溶剂交换是确保金属有机框架(MOF)完全活化的关键步骤;然而,MOF 之间的溶剂交换条件各不相同,甚至在同构变体中也是如此。本研究通过研究同构 M 2 (dobdc) (M=Mg, Co, Zn) 系列来研究影响溶剂交换的因素。使用常用溶剂 N,N-二甲基甲酰胺 (DMF)、乙醇 (EtOH) 和甲醇 (MeOH) 来评估 M 2 (dobdc) 配位不饱和位点 (CUS) 的溶剂交换。通过监测溶剂交换过程中 MOF 释放的溶剂以及 MOF 内金属的配位环境,可以构建出溶剂交换早期阶段的交换率以及最后痕量结合溶剂的排出情况。这种区分是通过溶剂相成分的整体监测和单晶水平上拉曼光谱的显微应用相结合来实现的。研究表明,溶剂置换的动力学对协同性有很大贡献;这种现象在正向和反向都可以观察到。热重分析与红外光谱和密度泛函理论 (DFT) 计算相结合,用于阐明溶剂交换速率和溶剂结合能之间的关系。溶剂交换速率由溶剂交换的动力学势垒决定,不遵循溶剂结合亲和力的顺序。这项工作通过研究结合强度、交换动力学和协同性之间的相互作用,有助于理解 MOF 的溶剂交换。它进一步为审查 MOF 激活协议提供了宝贵的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号