当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Antifungal Activities of Novel Potent Fluoroalkenyl Succinate Dehydrogenase Inhibitors
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-06-21 , DOI: 10.1021/acs.jafc.3c08693 Yu Chen 1 , Weilong Xu 1 , Mian Du 1 , Longzhu Bao 1 , Jun Li 1 , Qianqian Zhai 1 , Dingce Yan 2 , Huailong Teng 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-06-21 , DOI: 10.1021/acs.jafc.3c08693 Yu Chen 1 , Weilong Xu 1 , Mian Du 1 , Longzhu Bao 1 , Jun Li 1 , Qianqian Zhai 1 , Dingce Yan 2 , Huailong Teng 1
Affiliation
|
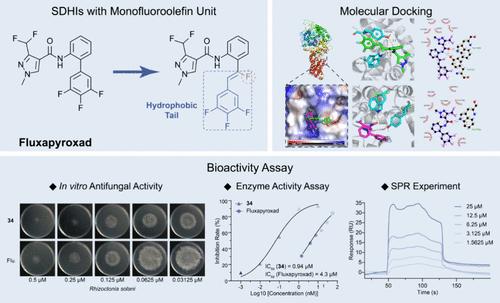
The development of new fungicide molecules is a crucial task for agricultural chemists to enhance the effectiveness of fungicides in agricultural production. In this study, a series of novel fluoroalkenyl modified succinate dehydrogenase inhibitors were synthesized and evaluated for their antifungal activities against eight fungi. The results from the in vitro antifungal assay demonstrated that compound 34 exhibited superior activity against Rhizoctonia solani with an EC50 value of 0.04 μM, outperforming commercial fluxapyroxad (EC50 = 0.18 μM) and boscalid (EC50 = 3.07 μM). Furthermore, compound 34 showed similar effects to fluxapyroxad on other pathogenic fungi such as Sclerotinia sclerotiorum (EC50 = 1.13 μM), Monilinia fructicola (EC50 = 1.61 μM), Botrytis cinerea (EC50 = 1.21 μM), and also demonstrated protective and curative efficacies in vivo on rapeseed leaves and tomato fruits. Enzyme activity experiments and protein–ligand interaction analysis by surface plasmon resonance revealed that compound 34 had a stronger inhibitory effect on succinate dehydrogenase compared to fluxapyroxad. Additionally, molecular docking and DFT calculation confirmed that the fluoroalkenyl unit in compound 34 could enhance its binding capacity with the target protein through p−π conjugation and hydrogen bond interactions.
中文翻译:

新型强效氟代烯基琥珀酸脱氢酶抑制剂的设计、合成和抗真菌活性
开发新型杀菌剂分子是农业化学家提高杀菌剂在农业生产中的有效性的一项重要任务。在这项研究中,合成了一系列新型氟代烯基修饰的琥珀酸脱氢酶抑制剂,并评估了它们对八种真菌的抗真菌活性。体外抗真菌试验结果表明,化合物34对立枯丝核菌表现出优异的活性,EC 50 值为0.04 μM,优于市售fluxapyroxad(EC 50 = 0.18 μM),啶酰菌胺 (EC 50 = 3.07 μM)。此外,化合物 34 对其他病原真菌(如核盘菌 (EC 50 = 1.13 μM)、Monilinia fructicola (EC 50 = 1.61 μM)、灰葡萄孢 (Botrytis cinerea) 表现出与 Fluxapyroxad 类似的作用。 EC 50 = 1.21 μM),并且在体内对油菜叶和番茄果实也表现出保护和治疗作用。酶活性实验和表面等离子共振蛋白质-配体相互作用分析表明,与fluxapyroxad相比,化合物34对琥珀酸脱氢酶具有更强的抑制作用。此外,分子对接和DFT计算证实,化合物34中的氟烯基单元可以通过p−π共轭和氢键相互作用增强其与目标蛋白的结合能力。
更新日期:2024-06-22
中文翻译:

新型强效氟代烯基琥珀酸脱氢酶抑制剂的设计、合成和抗真菌活性
开发新型杀菌剂分子是农业化学家提高杀菌剂在农业生产中的有效性的一项重要任务。在这项研究中,合成了一系列新型氟代烯基修饰的琥珀酸脱氢酶抑制剂,并评估了它们对八种真菌的抗真菌活性。体外抗真菌试验结果表明,化合物34对立枯丝核菌表现出优异的活性,EC 50 值为0.04 μM,优于市售fluxapyroxad(EC 50 = 0.18 μM),啶酰菌胺 (EC 50 = 3.07 μM)。此外,化合物 34 对其他病原真菌(如核盘菌 (EC 50 = 1.13 μM)、Monilinia fructicola (EC 50 = 1.61 μM)、灰葡萄孢 (Botrytis cinerea) 表现出与 Fluxapyroxad 类似的作用。 EC 50 = 1.21 μM),并且在体内对油菜叶和番茄果实也表现出保护和治疗作用。酶活性实验和表面等离子共振蛋白质-配体相互作用分析表明,与fluxapyroxad相比,化合物34对琥珀酸脱氢酶具有更强的抑制作用。此外,分子对接和DFT计算证实,化合物34中的氟烯基单元可以通过p−π共轭和氢键相互作用增强其与目标蛋白的结合能力。






































 京公网安备 11010802027423号
京公网安备 11010802027423号