当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Synthesis and Stereochemical Assignment of Enteropeptin A
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-23 , DOI: 10.1021/jacs.4c06126 Yiwei Zhang 1 , Shuvendu Saha 1 , Yannik C C Esser 1 , Chi P Ting 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-06-23 , DOI: 10.1021/jacs.4c06126 Yiwei Zhang 1 , Shuvendu Saha 1 , Yannik C C Esser 1 , Chi P Ting 1
Affiliation
|
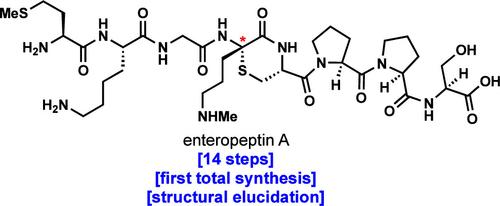
The total synthesis and structural elucidation of the antimicrobial sactipeptide enteropeptin A is reported. Enteropeptin A contains a thioaminoketal group with an unassigned stereochemical configuration that is embedded in a highly unusual thiomorpholine ring. In this synthesis, a linear peptide containing a dehydroamino acid and a pendant cysteine residue is subjected to Markovnikov hydrothiolation by a dithiophosphoric acid catalyst. This cyclization reaction forms the central thiomorpholine ring found in the enteropeptins. Both diastereomers at the unassigned thioaminoketal stereocenter of enteropeptin A were prepared, and their comparison to an authentic standard allowed for the unambiguous stereochemical assignment of the natural product to be of the D configuration. This inaugural total synthesis of enteropeptin A represents the first total synthesis of a sactipeptide reported to date. Moreover, the strategy disclosed herein serves as a general platform for the synthesis of stereochemically defined thiomorpholine-containing peptides, which may enable the discovery of new cyclic peptide antibiotics.
中文翻译:

肠肽A的全合成及立体化学归属
报道了抗菌肽肠肽 A 的全合成和结构阐明。肠肽 A 含有一个硫代氨基缩酮基团,该基团具有未指定的立体化学构型,嵌入在一个非常不寻常的硫代吗啉环中。在该合成中,含有脱氢氨基酸和侧链半胱氨酸残基的线性肽通过二硫代磷酸催化剂进行马尔可夫尼科夫氢硫基化。该环化反应形成肠肽素中的中心硫代吗啉环。制备了肠肽A未指定的硫代氨基缩酮立体中心处的两种非对映异构体,并将它们与真实标准品进行比较,允许将天然产物明确地立体化学指定为D构型。肠肽素 A 的首次全合成代表了迄今为止报道的第一个 sacipeptide 的全合成。此外,本文公开的策略充当合成立体化学定义的含硫代吗啉的肽的通用平台,这可能使得能够发现新的环肽抗生素。
更新日期:2024-06-23
中文翻译:

肠肽A的全合成及立体化学归属
报道了抗菌肽肠肽 A 的全合成和结构阐明。肠肽 A 含有一个硫代氨基缩酮基团,该基团具有未指定的立体化学构型,嵌入在一个非常不寻常的硫代吗啉环中。在该合成中,含有脱氢氨基酸和侧链半胱氨酸残基的线性肽通过二硫代磷酸催化剂进行马尔可夫尼科夫氢硫基化。该环化反应形成肠肽素中的中心硫代吗啉环。制备了肠肽A未指定的硫代氨基缩酮立体中心处的两种非对映异构体,并将它们与真实标准品进行比较,允许将天然产物明确地立体化学指定为D构型。肠肽素 A 的首次全合成代表了迄今为止报道的第一个 sacipeptide 的全合成。此外,本文公开的策略充当合成立体化学定义的含硫代吗啉的肽的通用平台,这可能使得能够发现新的环肽抗生素。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号