当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Arsenate reductase of Rufibacter tibetensis is a metallophosphoesterase evolved to catalyze redox reactions
Molecular Microbiology ( IF 2.6 ) Pub Date : 2024-06-22 , DOI: 10.1111/mmi.15289
Jie Shen 1 , Xin-Wei Song 2 , David Bickel 3, 4 , Barry P Rosen 5 , Fang-Jie Zhao 1 , Joris Messens 3, 6, 7 , Jun Zhang 1
Molecular Microbiology ( IF 2.6 ) Pub Date : 2024-06-22 , DOI: 10.1111/mmi.15289
Jie Shen 1 , Xin-Wei Song 2 , David Bickel 3, 4 , Barry P Rosen 5 , Fang-Jie Zhao 1 , Joris Messens 3, 6, 7 , Jun Zhang 1
Affiliation
|
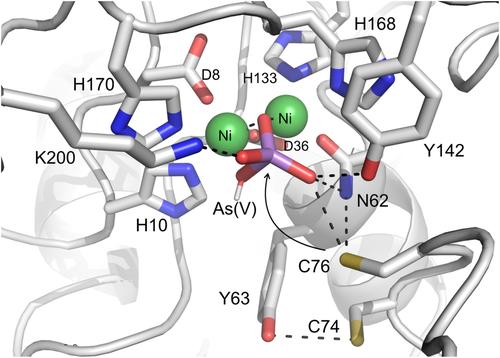
An arsenate reductase (Car1) from the Bacteroidetes species Rufibacter tibetensis 1351T was isolated from the Tibetan Plateau. The strain exhibits resistance to arsenite [As(III)] and arsenate [As(V)] and reduces As(V) to As(III). Here we shed light on the mechanism of enzymatic reduction by Car1. AlphaFold2 structure prediction, active site energy minimization, and steady-state kinetics of wild-type and mutant enzymes give insight into the catalytic mechanism. Car1 is structurally related to calcineurin-like metallophosphoesterases (MPPs). It functions as a binuclear metal hydrolase with limited phosphatase activity, particularly relying on the divalent metal Ni2+. As an As(V) reductase, it displays metal promiscuity and is coupled to the thioredoxin redox cycle, requiring the participation of two cysteine residues, Cys74 and Cys76. These findings suggest that Car1 evolved from a common ancestor of extant phosphatases by incorporating a redox function into an existing MPP catalytic site. Its proposed mechanism of arsenate reduction involves Cys74 initiating a nucleophilic attack on arsenate, leading to the formation of a covalent intermediate. Next, a nucleophilic attack of Cys76 leads to the release of As(III) and the formation of a surface-exposed Cys74-Cys76 disulfide, ready for reduction by thioredoxin.
中文翻译:

Rufibacter tibetensis 的砷酸还原酶是一种金属磷酸酯酶,旨在催化氧化还原反应
从青藏高原分离出来自拟杆菌属Rufibacter tibetensis 1351 T的砷酸还原酶 (Car1)。该菌株表现出对亚砷酸盐 [As(III)] 和砷酸盐 [As(V)] 的抗性,并将 As(V) 还原为 As(III)。在这里,我们阐明了 Car1 酶促还原的机制。 AlphaFold2 结构预测、活性位点能量最小化以及野生型和突变型酶的稳态动力学可以深入了解催化机制。 Car1 在结构上与钙调神经磷酸酶样金属磷酸酯酶 (MPP) 相关。它作为双核金属水解酶发挥作用,磷酸酶活性有限,特别依赖于二价金属 Ni 2+ 。作为一种 As(V) 还原酶,它表现出金属混杂性并与硫氧还蛋白氧化还原循环偶联,需要两个半胱氨酸残基 Cys74 和 Cys76 的参与。这些发现表明,Car1 通过将氧化还原功能整合到现有的 MPP 催化位点中,从现有磷酸酶的共同祖先进化而来。其提出的砷酸盐还原机制涉及 Cys74 引发对砷酸盐的亲核攻击,导致共价中间体的形成。接下来,Cys76 的亲核攻击导致 As(III) 的释放并形成表面暴露的 Cys74-Cys76 二硫化物,准备被硫氧还蛋白还原。
更新日期:2024-06-22
中文翻译:

Rufibacter tibetensis 的砷酸还原酶是一种金属磷酸酯酶,旨在催化氧化还原反应
从青藏高原分离出来自拟杆菌属Rufibacter tibetensis 1351 T的砷酸还原酶 (Car1)。该菌株表现出对亚砷酸盐 [As(III)] 和砷酸盐 [As(V)] 的抗性,并将 As(V) 还原为 As(III)。在这里,我们阐明了 Car1 酶促还原的机制。 AlphaFold2 结构预测、活性位点能量最小化以及野生型和突变型酶的稳态动力学可以深入了解催化机制。 Car1 在结构上与钙调神经磷酸酶样金属磷酸酯酶 (MPP) 相关。它作为双核金属水解酶发挥作用,磷酸酶活性有限,特别依赖于二价金属 Ni 2+ 。作为一种 As(V) 还原酶,它表现出金属混杂性并与硫氧还蛋白氧化还原循环偶联,需要两个半胱氨酸残基 Cys74 和 Cys76 的参与。这些发现表明,Car1 通过将氧化还原功能整合到现有的 MPP 催化位点中,从现有磷酸酶的共同祖先进化而来。其提出的砷酸盐还原机制涉及 Cys74 引发对砷酸盐的亲核攻击,导致共价中间体的形成。接下来,Cys76 的亲核攻击导致 As(III) 的释放并形成表面暴露的 Cys74-Cys76 二硫化物,准备被硫氧还蛋白还原。







































 京公网安备 11010802027423号
京公网安备 11010802027423号