当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficacy, tolerability and pharmacokinetics of survodutide, a glucagon/glucagon-like peptide-1 receptor dual agonist, in cirrhosis
Journal of Hepatology ( IF 26.8 ) Pub Date : 2024-06-08 , DOI: 10.1016/j.jhep.2024.06.003 Eric J Lawitz 1 , Mandy Fraessdorf 2 , Guy W Neff 3 , Jörn M Schattenberg 4 , Mazen Noureddin 5 , Naim Alkhouri 6 , Bernhard Schmid 7 , Charles P Andrews 8 , István Takács 9 , Samina Ajaz Hussain 2 , Wiebke K Fenske 10 , Edward J Gane 11 , Azadeh Hosseini-Tabatabaei 12 , Arun J Sanyal 13 , Daniel F Mazo 2 , Ramy Younes 2 ,
Journal of Hepatology ( IF 26.8 ) Pub Date : 2024-06-08 , DOI: 10.1016/j.jhep.2024.06.003 Eric J Lawitz 1 , Mandy Fraessdorf 2 , Guy W Neff 3 , Jörn M Schattenberg 4 , Mazen Noureddin 5 , Naim Alkhouri 6 , Bernhard Schmid 7 , Charles P Andrews 8 , István Takács 9 , Samina Ajaz Hussain 2 , Wiebke K Fenske 10 , Edward J Gane 11 , Azadeh Hosseini-Tabatabaei 12 , Arun J Sanyal 13 , Daniel F Mazo 2 , Ramy Younes 2 ,
Affiliation
|
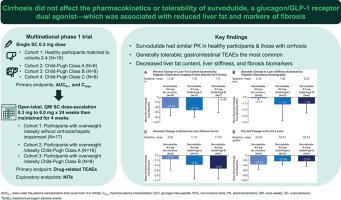
Survodutide is a glucagon/glucagon-like peptide-1 receptor dual agonist in development for the treatment of metabolic dysfunction-associated steatohepatitis (MASH). We investigated the pharmacokinetic and safety profile of survodutide in people with cirrhosis. This multinational, non-randomized, open-label, phase I clinical trial initially evaluated a single subcutaneous dose of survodutide 0.3 mg in people with Child-Pugh class A, B or C cirrhosis and healthy individuals with or without overweight/obesity matched for age, sex, and weight; the primary endpoints were the area under the plasma concentration-time curve from 0 to infinity (AUC) and maximal plasma concentration (C). Subsequently, people with overweight/obesity with or without cirrhosis (Child-Pugh class A or B) received once-weekly subcutaneous doses escalated from 0.3 mg to 6.0 mg over 24 weeks then maintained for 4 weeks; the primary endpoint was drug-related treatment-emergent adverse events, with MASH/cirrhosis-related endpoints explored. In the single-dose cohorts (n = 41), mean AUC and C were similar in those with cirrhosis compared with healthy individuals (90% CIs for adjusted geometric mean ratios spanned 1). Drug-related adverse events occurred in 25.0% of healthy individuals and ≤25.0% of those with cirrhosis after single doses, and 82.4% and 87.5%, respectively, of the multiple-dose cohorts (n = 41) over 28 weeks. Liver fat content, liver stiffness, liver volume, body weight, and other hepatic and metabolic disease markers were generally reduced after 28 weeks of survodutide treatment. Survodutide is generally tolerable in people with compensated or decompensated cirrhosis, does not require pharmacokinetic-related dose adjustment, and may improve liver-related non-invasive tests, supporting its investigation for MASH-related cirrhosis. Survodutide is a glucagon receptor/glucagon-like peptide-1 receptor dual agonist in development for treatment of metabolic dysfunction-associated steatohepatitis (MASH), which causes cirrhosis in ∼20% of cases. This trial delineates the pharmacokinetic and safety profile of survodutide in people with compensated or decompensated cirrhosis, and revealed associated reductions in liver fat content, markers of liver fibrosis and body weight. These findings have potential relevance for people with MASH—including those with decompensated cirrhosis, who are usually excluded from clinical trials of investigational drugs. Based on this study, further investigation of survodutide for MASH-related cirrhosis is warranted. NCT05296733.
中文翻译:

survodutide(一种胰高血糖素/胰高血糖样肽-1 受体双重激动剂)在肝硬化中的功效、耐受性和药代动力学
Survodutide 是一种胰高血糖素/胰高血糖样肽 1 受体双重激动剂,正在开发用于治疗代谢功能障碍相关的脂肪性肝炎 (MASH)。我们研究了 survodutide 在肝硬化患者中的药代动力学和安全性。这项跨国、非随机、开放标签的 I 期临床试验最初在 Child-Pugh A、B 或 C 级肝硬化患者以及年龄相匹配的超重/肥胖健康个体中评估了单次皮下注射 survodutide 0.3 mg 、性别和体重;主要终点是从0到无穷大的血浆浓度-时间曲线下面积(AUC)和最大血浆浓度(C)。随后,患有或不患有肝硬化的超重/肥胖患者(Child-Pugh A 级或 B 级)每周接受一次皮下注射,剂量在 24 周内从 0.3 mg 逐渐增加至 6.0 mg,然后维持 4 周;主要终点是与药物相关的治疗引起的不良事件,并探讨了与 MASH/肝硬化相关的终点。在单剂量队列 (n = 41) 中,肝硬化患者的平均 AUC 和 Cmax 与健康个体相似(调整几何平均比率的 90% CI 跨越 1)。 28 周内,单剂量给药后,25.0% 的健康个体和 ≤25.0% 的肝硬化个体发生药物相关不良事件,多剂量组 (n = 41) 的发生率分别为 82.4% 和 87.5%。 survodutide 治疗 28 周后,肝脏脂肪含量、肝脏硬度、肝脏体积、体重和其他肝脏和代谢疾病标志物普遍降低。 Survodutide 在代偿期或失代偿性肝硬化患者中通常是可耐受的,不需要药代动力学相关的剂量调整,并且可能改善肝脏相关的非侵入性测试,支持其对 MASH 相关肝硬化的研究。 Survodutide 是一种胰高血糖素受体/胰高血糖素样肽 1 受体双重激动剂,正在开发用于治疗代谢功能障碍相关的脂肪性肝炎 (MASH),约 20% 的病例会导致肝硬化。该试验描述了 survodutide 在代偿期或失代偿性肝硬化患者中的药代动力学和安全性,并揭示了肝脏脂肪含量、肝纤维化标志物和体重的相关降低。这些发现与 MASH 患者(包括失代偿性肝硬化患者)具有潜在相关性,他们通常被排除在研究药物的临床试验之外。基于这项研究,有必要对 survodutide 治疗 MASH 相关肝硬化进行进一步研究。 NCT05296733。
更新日期:2024-06-08
中文翻译:

survodutide(一种胰高血糖素/胰高血糖样肽-1 受体双重激动剂)在肝硬化中的功效、耐受性和药代动力学
Survodutide 是一种胰高血糖素/胰高血糖样肽 1 受体双重激动剂,正在开发用于治疗代谢功能障碍相关的脂肪性肝炎 (MASH)。我们研究了 survodutide 在肝硬化患者中的药代动力学和安全性。这项跨国、非随机、开放标签的 I 期临床试验最初在 Child-Pugh A、B 或 C 级肝硬化患者以及年龄相匹配的超重/肥胖健康个体中评估了单次皮下注射 survodutide 0.3 mg 、性别和体重;主要终点是从0到无穷大的血浆浓度-时间曲线下面积(AUC)和最大血浆浓度(C)。随后,患有或不患有肝硬化的超重/肥胖患者(Child-Pugh A 级或 B 级)每周接受一次皮下注射,剂量在 24 周内从 0.3 mg 逐渐增加至 6.0 mg,然后维持 4 周;主要终点是与药物相关的治疗引起的不良事件,并探讨了与 MASH/肝硬化相关的终点。在单剂量队列 (n = 41) 中,肝硬化患者的平均 AUC 和 Cmax 与健康个体相似(调整几何平均比率的 90% CI 跨越 1)。 28 周内,单剂量给药后,25.0% 的健康个体和 ≤25.0% 的肝硬化个体发生药物相关不良事件,多剂量组 (n = 41) 的发生率分别为 82.4% 和 87.5%。 survodutide 治疗 28 周后,肝脏脂肪含量、肝脏硬度、肝脏体积、体重和其他肝脏和代谢疾病标志物普遍降低。 Survodutide 在代偿期或失代偿性肝硬化患者中通常是可耐受的,不需要药代动力学相关的剂量调整,并且可能改善肝脏相关的非侵入性测试,支持其对 MASH 相关肝硬化的研究。 Survodutide 是一种胰高血糖素受体/胰高血糖素样肽 1 受体双重激动剂,正在开发用于治疗代谢功能障碍相关的脂肪性肝炎 (MASH),约 20% 的病例会导致肝硬化。该试验描述了 survodutide 在代偿期或失代偿性肝硬化患者中的药代动力学和安全性,并揭示了肝脏脂肪含量、肝纤维化标志物和体重的相关降低。这些发现与 MASH 患者(包括失代偿性肝硬化患者)具有潜在相关性,他们通常被排除在研究药物的临床试验之外。基于这项研究,有必要对 survodutide 治疗 MASH 相关肝硬化进行进一步研究。 NCT05296733。











































 京公网安备 11010802027423号
京公网安备 11010802027423号