当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of novel 4-substituted 7H-pyrrolo[2,3-d]pyrimidine derivatives as new FtsZ inhibitors: Bioactivity evaluation and computational simulation
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2024-06-05 , DOI: 10.1016/j.bioorg.2024.107534 Ting Li 1 , Ya Zhou 1 , Xichun Fu 1 , Linli Yang 1 , Hongwu Liu 1 , Xiang Zhou 1 , Liwei Liu 1 , Zhibing Wu 1 , Song Yang 1
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2024-06-05 , DOI: 10.1016/j.bioorg.2024.107534 Ting Li 1 , Ya Zhou 1 , Xichun Fu 1 , Linli Yang 1 , Hongwu Liu 1 , Xiang Zhou 1 , Liwei Liu 1 , Zhibing Wu 1 , Song Yang 1
Affiliation
|
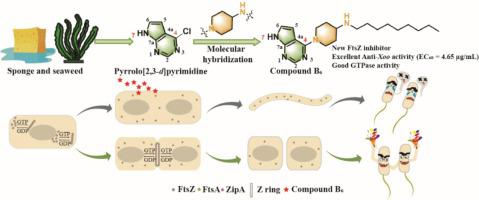
Bacterial infections and the consequent outburst of bactericide-resistance issues are fatal menace to both global health and agricultural produce. Hence, it is crucial to explore candidate bactericides with new mechanisms of action. The filamenting temperature-sensitive mutant Z (FtsZ) protein has been recognized as a new promising and effective target for new bactericide discovery. Hence, using a scaffold-hopping strategy, we designed new 7-pyrrolo[2,3-]pyrimidine derivatives, evaluated their antibacterial activities, and investigated their structure–activity relationships. Among them, compound exhibited the optimal bioactivity (EC = 4.65 µg/mL) against pv. (), which was superior to the references (bismerthiazol [BT], EC = 48.67 µg/mL; thiodiazole copper [TC], EC = 98.57 µg/mL]. Furthermore, the potency of compound in targeting FtsZ was validated by GTPase activity assay, FtsZ self-assembly observation, fluorescence titration, Fourier-transform infrared spectroscopy (FT-IR) assay, molecular dynamics simulations, and morphological observation. The GTPase activity assay showed that the final IC value of compound against FtsZ was 235.0 μM. Interestingly, the GTPase activity results indicated that the -FtsZ complex has an excellent binding constant ( = 10 M). Overall, the antibacterial behavior suggests that can interact with FtsZ and inhibit its GTPase activity, leading to bacterial cell elongation and even death. In addition, compound showed acceptable anti- activity and low toxicity, and also demonstrated a favorable pharmacokinetic profile predicted by ADMET analysis. Our findings provide new chemotypes for the development of FtsZ inhibitors as well as insights into their underlying mechanisms of action.
中文翻译:

新型 4-取代 7H-吡咯并[2,3-d]嘧啶衍生物作为新型 FtsZ 抑制剂的鉴定:生物活性评估和计算模拟
细菌感染和随之而来的杀菌剂耐药性问题的爆发对全球健康和农产品造成致命威胁。因此,探索具有新作用机制的候选杀菌剂至关重要。丝状温度敏感突变体 Z (FtsZ) 蛋白已被认为是新杀菌剂发现的新的有前景且有效的靶标。因此,利用支架跳跃策略,我们设计了新的7-吡咯并[2,3-]嘧啶衍生物,评估了它们的抗菌活性,并研究了它们的构效关系。其中,化合物对 pv 表现出最佳的生物活性(EC = 4.65 µg/mL)。 (),优于参考文献(双噻唑 [BT],EC = 48.67 µg/mL;噻二唑铜 [TC],EC = 98.57 µg/mL]。此外,化合物靶向 FtsZ 的效力通过 GTPase 活性进行了验证实验、FtsZ 自组装观察、荧光滴定、傅里叶变换红外光谱 (FT-IR) 测定、分子动力学模拟和形态学观察表明,化合物对 FtsZ 的最终 IC 值为 235.0 μM。 GTPase活性结果表明-FtsZ复合物具有优异的结合常数(= 10 M),总体而言,抗菌行为表明可以与FtsZ相互作用并抑制其GTPase活性,导致细菌细胞伸长甚至死亡。 ,化合物显示出可接受的抗活性和低毒性,并且还表现出 ADMET 分析预测的良好药代动力学特征,我们的研究结果为 FtsZ 抑制剂的开发提供了新的化学型,并深入了解了其潜在的作用机制。
更新日期:2024-06-05
中文翻译:

新型 4-取代 7H-吡咯并[2,3-d]嘧啶衍生物作为新型 FtsZ 抑制剂的鉴定:生物活性评估和计算模拟
细菌感染和随之而来的杀菌剂耐药性问题的爆发对全球健康和农产品造成致命威胁。因此,探索具有新作用机制的候选杀菌剂至关重要。丝状温度敏感突变体 Z (FtsZ) 蛋白已被认为是新杀菌剂发现的新的有前景且有效的靶标。因此,利用支架跳跃策略,我们设计了新的7-吡咯并[2,3-]嘧啶衍生物,评估了它们的抗菌活性,并研究了它们的构效关系。其中,化合物对 pv 表现出最佳的生物活性(EC = 4.65 µg/mL)。 (),优于参考文献(双噻唑 [BT],EC = 48.67 µg/mL;噻二唑铜 [TC],EC = 98.57 µg/mL]。此外,化合物靶向 FtsZ 的效力通过 GTPase 活性进行了验证实验、FtsZ 自组装观察、荧光滴定、傅里叶变换红外光谱 (FT-IR) 测定、分子动力学模拟和形态学观察表明,化合物对 FtsZ 的最终 IC 值为 235.0 μM。 GTPase活性结果表明-FtsZ复合物具有优异的结合常数(= 10 M),总体而言,抗菌行为表明可以与FtsZ相互作用并抑制其GTPase活性,导致细菌细胞伸长甚至死亡。 ,化合物显示出可接受的抗活性和低毒性,并且还表现出 ADMET 分析预测的良好药代动力学特征,我们的研究结果为 FtsZ 抑制剂的开发提供了新的化学型,并深入了解了其潜在的作用机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号