当前位置:
X-MOL 学术
›
Clin. Gastroenterol. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development and Validation of a Scoring System to Predict Response to Obeticholic Acid in Primary Biliary Cholangitis
Clinical Gastroenterology and Hepatology ( IF 11.6 ) Pub Date : 2024-05-22 , DOI: 10.1016/j.cgh.2024.05.008 Antonio De Vincentis 1 , Javier Ampuero 2 , Francesca Terracciani 3 , Daphne D'Amato 4 , Alessio Gerussi 5 , Laura Cristoferi 5 , Nora Cazzagon 6 , Emanuela Bonaiuto 6 , Annarosa Floreani 6 , Vincenza Calvaruso 7 , Luca Cadamuro 7 , Elisabetta Degasperi 8 , Anna Morgando 4 , Ester Vanni 4 , Ana Lleo 9 , Francesca Colapietro 9 , Domenico Alvaro 10 , Antonino Castellaneta 11 , Sara Labanca 12 , Mauro Viganò 13 , Marco Distefano 14 , Valeria Pace Palitti 15 , Chiara Ricci 16 , Nicoletta De Matthaeis 17 , Marco Marzioni 18 , Elena Gómez-Dominguez 19 , Jose-Luis Montero 20 , Esther Molina 21 , Luisa Garcia-Buey 22 , Marta Casado 23 , Marina Berenguer 24 , Isabel Conde 24 , Miguel-Angel Simon 25 , Javier Fuentes 26 , Pedro Costa-Moreira 27 , Guilherme Macedo 27 , Francisco Jorquera 28 , Rosa-Maria Morillas 29 , Jose Presa 30 , Jose-Manuel Sousa 2 , Dario Gomes 31 , Luis Santos 31 , Antonio Olveira 32 , Manuel Hernandez-Guerra 33 , Leire Aburruza 34 , Arsenio Santos 35 , Armando Carvalho 35 , Juan Uriz 36 , Maria-Luisa Gutierrez 37 , Elia Perez 37 , Luchino Chessa 38 , Adriano Pellicelli 39 , Massimo Marignani 40 , Luigi Muratori 41 , Grazia Anna Niro 42 , Maurizia Brunetto 43 , Francesca Romana Ponziani 44 , Maurizio Pompili 44 , Fabio Marra 45 , Andrea Galli 45 , Alessandro Mussetto 46 , Giuliano Alagna 47 , Loredana Simone 48 , Gaetano Bertino 49 , Floriano Rosina 50 , Raffaele Cozzolongo 51 , Maurizio Russello 52 , Leonardo Baiocchi 53 , Carlo Saitta 54 , Natalia Terreni 55 , Teresa Zolfino 56 , Cristina Rigamonti 57 , Raffaella Vigano 58 , Giuseppe Cuccorese 59 , Pietro Pozzoni 60 , Claudio Pedone 61 , Simone Grasso 62 , Antonio Picardi 3 , Pietro Invernizzi 5 , Rodolfo Sacco 63 , Antonio Izzi 64 , Conrado Fernandez-Rodriguez 65 , Umberto Vespasiani-Gentilucci 3 , Marco Carbone 5 ,
Clinical Gastroenterology and Hepatology ( IF 11.6 ) Pub Date : 2024-05-22 , DOI: 10.1016/j.cgh.2024.05.008 Antonio De Vincentis 1 , Javier Ampuero 2 , Francesca Terracciani 3 , Daphne D'Amato 4 , Alessio Gerussi 5 , Laura Cristoferi 5 , Nora Cazzagon 6 , Emanuela Bonaiuto 6 , Annarosa Floreani 6 , Vincenza Calvaruso 7 , Luca Cadamuro 7 , Elisabetta Degasperi 8 , Anna Morgando 4 , Ester Vanni 4 , Ana Lleo 9 , Francesca Colapietro 9 , Domenico Alvaro 10 , Antonino Castellaneta 11 , Sara Labanca 12 , Mauro Viganò 13 , Marco Distefano 14 , Valeria Pace Palitti 15 , Chiara Ricci 16 , Nicoletta De Matthaeis 17 , Marco Marzioni 18 , Elena Gómez-Dominguez 19 , Jose-Luis Montero 20 , Esther Molina 21 , Luisa Garcia-Buey 22 , Marta Casado 23 , Marina Berenguer 24 , Isabel Conde 24 , Miguel-Angel Simon 25 , Javier Fuentes 26 , Pedro Costa-Moreira 27 , Guilherme Macedo 27 , Francisco Jorquera 28 , Rosa-Maria Morillas 29 , Jose Presa 30 , Jose-Manuel Sousa 2 , Dario Gomes 31 , Luis Santos 31 , Antonio Olveira 32 , Manuel Hernandez-Guerra 33 , Leire Aburruza 34 , Arsenio Santos 35 , Armando Carvalho 35 , Juan Uriz 36 , Maria-Luisa Gutierrez 37 , Elia Perez 37 , Luchino Chessa 38 , Adriano Pellicelli 39 , Massimo Marignani 40 , Luigi Muratori 41 , Grazia Anna Niro 42 , Maurizia Brunetto 43 , Francesca Romana Ponziani 44 , Maurizio Pompili 44 , Fabio Marra 45 , Andrea Galli 45 , Alessandro Mussetto 46 , Giuliano Alagna 47 , Loredana Simone 48 , Gaetano Bertino 49 , Floriano Rosina 50 , Raffaele Cozzolongo 51 , Maurizio Russello 52 , Leonardo Baiocchi 53 , Carlo Saitta 54 , Natalia Terreni 55 , Teresa Zolfino 56 , Cristina Rigamonti 57 , Raffaella Vigano 58 , Giuseppe Cuccorese 59 , Pietro Pozzoni 60 , Claudio Pedone 61 , Simone Grasso 62 , Antonio Picardi 3 , Pietro Invernizzi 5 , Rodolfo Sacco 63 , Antonio Izzi 64 , Conrado Fernandez-Rodriguez 65 , Umberto Vespasiani-Gentilucci 3 , Marco Carbone 5 ,
Affiliation
|
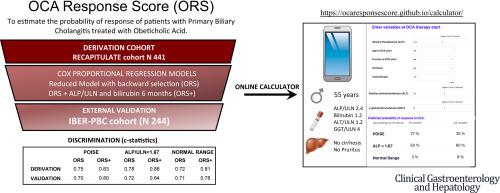
Obeticholic acid (OCA) is the only licensed second-line therapy for primary biliary cholangitis (PBC). With novel therapeutics in advanced development, clinical tools are needed to tailor the treatment algorithm. We aimed to derive and externally validate the OCA response score (ORS) for predicting the response probability of individuals with PBC to OCA. We used data from the Italian RECAPITULATE (N = 441) and the IBER-PBC (N = 244) OCA real-world prospective cohorts to derive/validate a score including widely available variables obtained either pre-treatment (ORS) or also after 6 months of treatment (ORS+). Multivariable Cox regressions with backward selection were applied to obtain parsimonious predictive models. The predicted outcomes were biochemical response according to POISE (alkaline phosphatase [ALP]/upper limit of normal [ULN]<1.67 with a reduction of at least 15%, and normal bilirubin), or ALP/ULN<1.67, or normal range criteria (NR: normal ALP, alanine aminotransferase [ALT], and bilirubin) up to 24 months. Depending on the response criteria, ORS included age, pruritus, cirrhosis, ALP/ULN, ALT/ULN, GGT/ULN, and bilirubin. ORS+ also included ALP/ULN and bilirubin after 6 months of OCA therapy. Internally validated c-statistics for ORS were 0.75, 0.78, and 0.72 for POISE, ALP/ULN<1.67, and NR response, which raised to 0.83, 0.88, and 0.81 with ORS+, respectively. The respective performances in validation were 0.70, 0.72, and 0.71 for ORS and 0.80, 0.84, and 0.78 for ORS+. Results were consistent across groups with mild/severe disease. We developed and externally validated a scoring system capable to predict OCA response according to different criteria. This tool will enhance a stratified second-line therapy model to streamline standard care and trial delivery in PBC.
中文翻译:

预测原发性胆汁性胆管炎奥贝胆酸反应的评分系统的开发和验证
奥贝胆酸(OCA)是唯一获得许可的原发性胆汁性胆管炎(PBC)二线治疗药物。随着新疗法的先进开发,需要临床工具来定制治疗算法。我们的目的是推导并在外部验证 OCA 反应评分 (ORS),以预测 PBC 患者对 OCA 的反应概率。我们使用来自意大利 RECAPITULATE (N = 441) 和 IBER-PBC (N = 244) OCA 现实世界前瞻性队列的数据来推导/验证评分,包括治疗前 (ORS) 或治疗后 6 小时获得的广泛可用变量治疗数月(ORS+)。应用具有向后选择的多变量 Cox 回归来获得简约的预测模型。预测结果是根据 POISE(碱性磷酸酶 [ALP]/正常上限 [ULN]<1.67,减少至少 15%,胆红素正常)或 ALP/ULN<1.67 的生化反应,或正常范围标准(NR:ALP、丙氨酸氨基转移酶 [ALT] 和胆红素正常)长达 24 个月。根据反应标准,ORS 包括年龄、瘙痒、肝硬化、ALP/ULN、ALT/ULN、GGT/ULN 和胆红素。 OCA 治疗 6 个月后,ORS+ 还包括 ALP/ULN 和胆红素。 POISE、ALP/ULN<1.67 和 NR 响应的 ORS 内部验证 c 统计量分别为 0.75、0.78 和 0.72,ORS+ 分别提高到 0.83、0.88 和 0.81。 ORS 的验证性能分别为 0.70、0.72 和 0.71,ORS+ 的验证性能分别为 0.80、0.84 和 0.78。轻度/重度疾病组的结果是一致的。我们开发并在外部验证了一个评分系统,能够根据不同的标准预测 OCA 反应。 该工具将增强分层二线治疗模型,以简化 PBC 的标准护理和试验实施。
更新日期:2024-05-22
中文翻译:

预测原发性胆汁性胆管炎奥贝胆酸反应的评分系统的开发和验证
奥贝胆酸(OCA)是唯一获得许可的原发性胆汁性胆管炎(PBC)二线治疗药物。随着新疗法的先进开发,需要临床工具来定制治疗算法。我们的目的是推导并在外部验证 OCA 反应评分 (ORS),以预测 PBC 患者对 OCA 的反应概率。我们使用来自意大利 RECAPITULATE (N = 441) 和 IBER-PBC (N = 244) OCA 现实世界前瞻性队列的数据来推导/验证评分,包括治疗前 (ORS) 或治疗后 6 小时获得的广泛可用变量治疗数月(ORS+)。应用具有向后选择的多变量 Cox 回归来获得简约的预测模型。预测结果是根据 POISE(碱性磷酸酶 [ALP]/正常上限 [ULN]<1.67,减少至少 15%,胆红素正常)或 ALP/ULN<1.67 的生化反应,或正常范围标准(NR:ALP、丙氨酸氨基转移酶 [ALT] 和胆红素正常)长达 24 个月。根据反应标准,ORS 包括年龄、瘙痒、肝硬化、ALP/ULN、ALT/ULN、GGT/ULN 和胆红素。 OCA 治疗 6 个月后,ORS+ 还包括 ALP/ULN 和胆红素。 POISE、ALP/ULN<1.67 和 NR 响应的 ORS 内部验证 c 统计量分别为 0.75、0.78 和 0.72,ORS+ 分别提高到 0.83、0.88 和 0.81。 ORS 的验证性能分别为 0.70、0.72 和 0.71,ORS+ 的验证性能分别为 0.80、0.84 和 0.78。轻度/重度疾病组的结果是一致的。我们开发并在外部验证了一个评分系统,能够根据不同的标准预测 OCA 反应。 该工具将增强分层二线治疗模型,以简化 PBC 的标准护理和试验实施。











































 京公网安备 11010802027423号
京公网安备 11010802027423号