当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hippo-YAP/TAZ-ROS signaling axis regulates metaflammation induced by SelenoM deficiency in high-fat diet-derived obesity
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-06-13 , DOI: 10.1016/j.jare.2024.06.005 Jingzeng Cai , Jiaqiang Huang , Di Li , Xintong Zhang , Bendong Shi , Qiaohan Liu , Cheng Fang , Shiwen Xu , Ziwei Zhang
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-06-13 , DOI: 10.1016/j.jare.2024.06.005 Jingzeng Cai , Jiaqiang Huang , Di Li , Xintong Zhang , Bendong Shi , Qiaohan Liu , Cheng Fang , Shiwen Xu , Ziwei Zhang
|
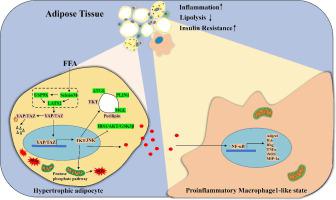
Metabolic inflammation (metaflammation) in obesity is primarily initiated by proinflammatory macrophage infiltration into adipose tissue. SelenoM contributes to the modulation of antioxidative stress and inflammation in multiple pathological processes; however, its roles in metaflammation and the proinflammatory macrophage (M1)-like state in adipose tissue have not been determined. We hypothesize that SelenoM could effectively regulate metaflammation via the Hippo-YAP/TAZ-ROS signaling axis in obesity derived from a high-fat diet. Morphological changes in adipose tissue were examined by hematoxylin-eosin (H&E) staining and fluorescence microscopy. The glucose tolerance test (GTT) and insulin tolerance test (ITT) were used to evaluate the impact of SelenoM deficiency on blood glucose levels. RNA-Seq analysis, LC-MS analysis, Mass spectrometry analysis and western blotting were performed to detect the levels of genes and proteins related to glycolipid metabolism in adipose tissue. Herein, we evaluated the inflammatory features and metabolic microenvironment of mice with SelenoM-deficient adipose tissues by multi-omics analyses. The deletion of SelenoM resulted in glycolipid metabolic disturbances and insulin resistance, thereby accelerating weight gain, adiposity, and hyperglycemia. Mice lacking SelenoM in white adipocytes developed severe adipocyte hypertrophy via impaired lipolysis. SelenoM deficiency aggravated the generation of ROS by reducing equivalents (NADPH and glutathione) in adipocytes, thereby promoting inflammatory cytokine production and the M1-proinflammatory reaction, which was related to a change in nuclear factor kappa-B (NF-κB) levels in macrophages. Mechanistically, SelenoM deficiency promoted metaflammation via Hippo-YAP/TAZ-ROS-mediated transcriptional regulation by targeting large tumor suppressor 2 (LATS2). Moreover, supplementation with N-acetyl cysteine (NAC) to reduce excessive oxidative stress partially rescued adipocyte inflammatory responses and macrophage M1 activation. Our data indicate that SelenoM ameliorates metaflammation mainly via the Hippo-YAP/TAZ-ROS signaling axis in obesity. The identification of SelenoM as a key regulator of metaflammation presents opportunities for the development of novel therapeutic interventions targeting adipose tissue dysfunction in obesity.
中文翻译:

Hippo-YAP/TAZ-ROS 信号轴调节高脂饮食源性肥胖中 SelenoM 缺乏引起的代谢炎症
肥胖中的代谢炎症(代谢炎症)主要是由促炎巨噬细胞浸润到脂肪组织中引发的。 SelenoM 有助于调节多种病理过程中的抗氧化应激和炎症;然而,其在脂肪组织中的代谢炎症和促炎巨噬细胞(M1)样状态中的作用尚未确定。我们假设 SelenoM 可以通过 Hippo-YAP/TAZ-ROS 信号轴有效调节高脂饮食引起的肥胖症的代谢炎症。通过苏木精-伊红 (H&E) 染色和荧光显微镜检查脂肪组织的形态变化。采用葡萄糖耐量试验(GTT)和胰岛素耐量试验(ITT)来评估SelenoM缺乏对血糖水平的影响。通过RNA-Seq分析、LC-MS分析、质谱分析和蛋白质印迹来检测脂肪组织中与糖脂代谢相关的基因和蛋白质的水平。在此,我们通过多组学分析评估了 SelenoM 脂肪组织缺陷小鼠的炎症特征和代谢微环境。 SelenoM 的缺失会导致糖脂代谢紊乱和胰岛素抵抗,从而加速体重增加、肥胖和高血糖。白色脂肪细胞中缺乏 SelenoM 的小鼠由于脂肪分解受损而出现严重的脂肪细胞肥大。 SelenoM 缺乏通过减少脂肪细胞中的当量(NADPH 和谷胱甘肽)来加剧 ROS 的产生,从而促进炎症细胞因子的产生和 M1 促炎症反应,这与巨噬细胞中核因子 kappa-B (NF-κB) 水平的变化有关。 从机制上讲,SelenoM 缺陷通过 Hippo-YAP/TAZ-ROS 介导的转录调节,通过靶向大肿瘤抑制因子 2 (LATS2) 促进代谢炎症。此外,补充 N-乙酰半胱氨酸 (NAC) 以减少过度氧化应激,部分挽救了脂肪细胞炎症反应和巨噬细胞 M1 活化。我们的数据表明,SelenoM 主要通过 Hippo-YAP/TAZ-ROS 信号轴改善肥胖症的代谢炎症。 SelenoM 被鉴定为代谢炎症的关键调节因子,为开发针对肥胖症脂肪组织功能障碍的新型治疗干预措施提供了机会。
更新日期:2024-06-13
中文翻译:

Hippo-YAP/TAZ-ROS 信号轴调节高脂饮食源性肥胖中 SelenoM 缺乏引起的代谢炎症
肥胖中的代谢炎症(代谢炎症)主要是由促炎巨噬细胞浸润到脂肪组织中引发的。 SelenoM 有助于调节多种病理过程中的抗氧化应激和炎症;然而,其在脂肪组织中的代谢炎症和促炎巨噬细胞(M1)样状态中的作用尚未确定。我们假设 SelenoM 可以通过 Hippo-YAP/TAZ-ROS 信号轴有效调节高脂饮食引起的肥胖症的代谢炎症。通过苏木精-伊红 (H&E) 染色和荧光显微镜检查脂肪组织的形态变化。采用葡萄糖耐量试验(GTT)和胰岛素耐量试验(ITT)来评估SelenoM缺乏对血糖水平的影响。通过RNA-Seq分析、LC-MS分析、质谱分析和蛋白质印迹来检测脂肪组织中与糖脂代谢相关的基因和蛋白质的水平。在此,我们通过多组学分析评估了 SelenoM 脂肪组织缺陷小鼠的炎症特征和代谢微环境。 SelenoM 的缺失会导致糖脂代谢紊乱和胰岛素抵抗,从而加速体重增加、肥胖和高血糖。白色脂肪细胞中缺乏 SelenoM 的小鼠由于脂肪分解受损而出现严重的脂肪细胞肥大。 SelenoM 缺乏通过减少脂肪细胞中的当量(NADPH 和谷胱甘肽)来加剧 ROS 的产生,从而促进炎症细胞因子的产生和 M1 促炎症反应,这与巨噬细胞中核因子 kappa-B (NF-κB) 水平的变化有关。 从机制上讲,SelenoM 缺陷通过 Hippo-YAP/TAZ-ROS 介导的转录调节,通过靶向大肿瘤抑制因子 2 (LATS2) 促进代谢炎症。此外,补充 N-乙酰半胱氨酸 (NAC) 以减少过度氧化应激,部分挽救了脂肪细胞炎症反应和巨噬细胞 M1 活化。我们的数据表明,SelenoM 主要通过 Hippo-YAP/TAZ-ROS 信号轴改善肥胖症的代谢炎症。 SelenoM 被鉴定为代谢炎症的关键调节因子,为开发针对肥胖症脂肪组织功能障碍的新型治疗干预措施提供了机会。






































 京公网安备 11010802027423号
京公网安备 11010802027423号