Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly efficient and selective hydrogenation of furfural to furfuryl alcohol and cyclopentanone over Cu-Ni bimetallic Catalysts: The crucial role of CuNi alloys and Cu+ species
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-06-13 , DOI: 10.1016/j.jcat.2024.115603 Xiaoqing Liao , Hao Zhao , Ruizhuo Liu , Hean Luo , Yang Lv , Pingle Liu
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-06-13 , DOI: 10.1016/j.jcat.2024.115603 Xiaoqing Liao , Hao Zhao , Ruizhuo Liu , Hean Luo , Yang Lv , Pingle Liu
|
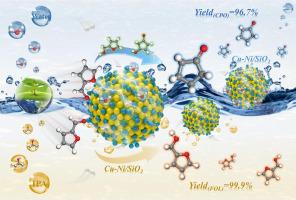
The selective hydrogenation of furfural derived from biomass is of significant importance in the synthesis of valuable chemical compounds. In this work, Cu-Ni bimetallic catalysts with varying metal ratios were effectively synthesized using the urea deposition method and employed in catalytic hydrogenation of furfural (FA) to furfuryl alcohol (FOL) and cyclopentanone (CPO) in different solvents. The study revealed that the synergistic interaction between Cu and Ni favors the formation of CuNi alloys, thereby facilitating the reduction of CuO and NiO and resulting in larger amount of metallic species (Cu and Ni), and promoting the formation of highly dispersed nanoparticles. Moreover, the formed Cu species can serve as Lewis acid sites and improve the adsorption of polarized C = O, thus significantly enhancing the selectivity to FOL. More importantly, the cooperation between Cu species and water can promote the aqueous-phase hydrogenation-rearrangement (AP-HR) of FA to CPO. Also, DRIFTS shows that CuNi/SiO preferentially adsorbs and activates C = O and prefers to form 4-hydroxy-2-cyclopentenone (HCP), which is the key intermediate to form CPO. DFT calculations agree with the DRIFTS, showing that CuNi (111) crystal surface with the lowest -band center and the strongest electronic effects presents the highest adsorption energies of H, HO, and FA, the lowest adsorption energies of H*, FOL, and CPO, and the lowest energy barriers of Piancatelli rearrangement of FOL to HCP. Under the optimum conditions, CuNi/SiO gives 99.9 % yield to FOL at 333 K in isopropanol and 96.7 % yield to CPO at 413 K in water. The low cost and high activity of CuNi/SiO make it a potential catalyst for the green production of FOL and CPO in industry.
中文翻译:

Cu-Ni 双金属催化剂上糠醛高效选择性加氢生成糠醇和环戊酮:CuNi 合金和 Cu+ 物质的关键作用
来自生物质的糠醛的选择性加氢对于合成有价值的化合物具有重要意义。在这项工作中,采用尿素沉积法有效合成了不同金属比例的Cu-Ni双金属催化剂,并将其用于不同溶剂中糠醛(FA)催化加氢生成糠醇(FOL)和环戊酮(CPO)。研究表明,Cu和Ni之间的协同相互作用有利于CuNi合金的形成,从而促进CuO和NiO的还原,产生大量的金属物质(Cu和Ni),并促进高度分散的纳米颗粒的形成。此外,形成的Cu物种可以作为路易斯酸位点并改善极化C = O的吸附,从而显着提高FOL的选择性。更重要的是,Cu物种与水之间的合作可以促进FA到CPO的水相氢化重排(AP-HR)。此外,DRIFTS 显示 CuNi/SiO 优先吸附和激活 C = O,并优先形成 4-羟基-2-环戊烯酮 (HCP),这是形成 CPO 的关键中间体。 DFT计算与DRIFTS一致,表明带中心最低、电子效应最强的CuNi(111)晶体表面对H、H2O和FA的吸附能最高,对H*、FOL和FA的吸附能最低。 CPO,以及 FOL 至 HCP 的 Piancatelli 重排的最低能垒。在最佳条件下,CuNi/SiO 在 333 K 的异丙醇中产生 99.9% 的 FOL,在 413 K 的水中产生 96.7% 的 CPO。 CuNi/SiO的低成本和高活性使其成为工业上FOL和CPO绿色生产的潜在催化剂。
更新日期:2024-06-13
中文翻译:

Cu-Ni 双金属催化剂上糠醛高效选择性加氢生成糠醇和环戊酮:CuNi 合金和 Cu+ 物质的关键作用
来自生物质的糠醛的选择性加氢对于合成有价值的化合物具有重要意义。在这项工作中,采用尿素沉积法有效合成了不同金属比例的Cu-Ni双金属催化剂,并将其用于不同溶剂中糠醛(FA)催化加氢生成糠醇(FOL)和环戊酮(CPO)。研究表明,Cu和Ni之间的协同相互作用有利于CuNi合金的形成,从而促进CuO和NiO的还原,产生大量的金属物质(Cu和Ni),并促进高度分散的纳米颗粒的形成。此外,形成的Cu物种可以作为路易斯酸位点并改善极化C = O的吸附,从而显着提高FOL的选择性。更重要的是,Cu物种与水之间的合作可以促进FA到CPO的水相氢化重排(AP-HR)。此外,DRIFTS 显示 CuNi/SiO 优先吸附和激活 C = O,并优先形成 4-羟基-2-环戊烯酮 (HCP),这是形成 CPO 的关键中间体。 DFT计算与DRIFTS一致,表明带中心最低、电子效应最强的CuNi(111)晶体表面对H、H2O和FA的吸附能最高,对H*、FOL和FA的吸附能最低。 CPO,以及 FOL 至 HCP 的 Piancatelli 重排的最低能垒。在最佳条件下,CuNi/SiO 在 333 K 的异丙醇中产生 99.9% 的 FOL,在 413 K 的水中产生 96.7% 的 CPO。 CuNi/SiO的低成本和高活性使其成为工业上FOL和CPO绿色生产的潜在催化剂。












































 京公网安备 11010802027423号
京公网安备 11010802027423号