当前位置:
X-MOL 学术
›
Redox Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Extracellular vesicles from II trimester human amniotic fluid as paracrine conveyors counteracting oxidative stress
Redox Biology ( IF 10.7 ) Pub Date : 2024-06-17 , DOI: 10.1016/j.redox.2024.103241 Giorgia Senesi 1 , Laura Guerricchio 2 , Maddalena Ghelardoni 3 , Nadia Bertola 3 , Stefano Rebellato 4 , Nicole Grinovero 5 , Martina Bartolucci 5 , Ambra Costa 3 , Andrea Raimondi 6 , Cristina Grange 7 , Sara Bolis 8 , Valentina Massa 9 , Dario Paladini 10 , Domenico Coviello 11 , Assunta Pandolfi 12 , Benedetta Bussolati 13 , Andrea Petretto 5 , Grazia Fazio 4 , Silvia Ravera 2 , Lucio Barile 1 , Carolina Balbi 14 , Sveva Bollini 15
Redox Biology ( IF 10.7 ) Pub Date : 2024-06-17 , DOI: 10.1016/j.redox.2024.103241 Giorgia Senesi 1 , Laura Guerricchio 2 , Maddalena Ghelardoni 3 , Nadia Bertola 3 , Stefano Rebellato 4 , Nicole Grinovero 5 , Martina Bartolucci 5 , Ambra Costa 3 , Andrea Raimondi 6 , Cristina Grange 7 , Sara Bolis 8 , Valentina Massa 9 , Dario Paladini 10 , Domenico Coviello 11 , Assunta Pandolfi 12 , Benedetta Bussolati 13 , Andrea Petretto 5 , Grazia Fazio 4 , Silvia Ravera 2 , Lucio Barile 1 , Carolina Balbi 14 , Sveva Bollini 15
Affiliation
|
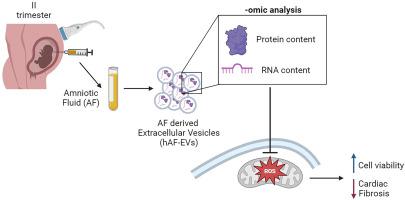
We previously demonstrated that the human amniotic fluid (hAF) from II trimester of gestation is a feasible source of stromal progenitors (human amniotic fluid stem cells, hAFSC), with significant paracrine potential for regenerative medicine. Extracellular vesicles (EVs) separated and concentrated from hAFSC secretome can deliver pro-survival, proliferative, anti-fibrotic and cardioprotective effects in preclinical models of skeletal and cardiac muscle injury. While hAFSC-EVs isolation can be significantly influenced by in vitro cell culture, here we profiled EVs directly concentrated from hAF as an alternative option and investigated their paracrine potential against oxidative stress. II trimester hAF samples were obtained as leftover material from prenatal diagnostic amniocentesis following written informed consent. EVs were separated by size exclusion chromatography and concentrated by ultracentrifugation. hAF-EVs were assessed by nanoparticle tracking analysis, transmission electron microscopy, Western Blot, and flow cytometry; their metabolic activity was evaluated by oximetric and luminometric analyses and their cargo profiled by proteomics and RNA sequencing. hAF-EV paracrine potential was tested in preclinical in vitro models of oxidative stress and dysfunction on murine C2C12 cells and on 3D human cardiac microtissue. Our protocol resulted in a yield of 6.31 ± 0.98 × 10 EVs particles per hAF milliliter showing round cup-shaped morphology and 209.63 ± 6.10 nm average size, with relevant expression of CD81, CD63 and CD9 tetraspanin markers. hAF-EVs were enriched in CD133/1, CD326, CD24, CD29, and SSEA4 and able to produce ATP by oxygen consumption. While oxidative stress significantly reduced C2C12 survival, hAF-EV priming resulted in significant rescue of cell viability, with notable recovery of ATP synthesis and concomitant reduction of cell damage and lipid peroxidation activity. 3D human cardiac microtissues treated with hAF-EVs and experiencing HO stress and TGFβ stimulation showed improved survival with a remarkable decrease in the onset of fibrosis. Our results suggest that leftover samples of II trimester human amniotic fluid can represent a feasible source of EVs to counteract oxidative damage on target cells, thus offering a novel candidate therapeutic option to counteract skeletal and cardiac muscle injury.
中文翻译:

来自妊娠中期人类羊水的细胞外囊泡作为旁分泌输送器对抗氧化应激
我们之前证明,妊娠第二个月的人羊水(hAF)是基质祖细胞(人羊水干细胞,hAFSC)的可行来源,具有再生医学的显着旁分泌潜力。从 hAFSC 分泌蛋白组中分离和浓缩的细胞外囊泡 (EV) 可以在骨骼和心肌损伤的临床前模型中发挥促生存、增殖、抗纤维化和心脏保护作用。虽然 hAFSC-EV 分离可能受到体外细胞培养的显着影响,但在这里我们分析了直接从 hAF 浓缩的 EV 作为替代选择,并研究了它们对抗氧化应激的旁分泌潜力。妊娠中期 hAF 样本是在书面知情同意后作为产前诊断羊膜穿刺术的剩余材料获得的。通过尺寸排阻色谱分离 EV,并通过超速离心浓缩。通过纳米颗粒跟踪分析、透射电子显微镜、蛋白质印迹和流式细胞术评估 hAF-EV;通过血氧测定和发光分析评估它们的代谢活性,并通过蛋白质组学和 RNA 测序对它们的货物进行分析。在小鼠 C2C12 细胞和 3D 人类心脏微组织的氧化应激和功能障碍的临床前体外模型中测试了 hAF-EV 旁分泌潜力。我们的方案产生了每 hAF 毫升 6.31 ± 0.98 × 10 EVs 颗粒的产量,显示圆杯形形态和 209.63 ± 6.10 nm 平均尺寸,并具有 CD81、CD63 和 CD9 四跨膜蛋白标记物的相关表达。 hAF-EV 富含 CD133/1、CD326、CD24、CD29 和 SSEA4,并且能够通过耗氧产生 ATP。 虽然氧化应激显着降低了 C2C12 的存活率,但 hAF-EV 引发可显着挽救细胞活力,显着恢复 ATP 合成,同时减少细胞损伤和脂质过氧化活性。用 hAF-EV 处理并经历 H2O2 应激和 TGFβ 刺激的 3D 人类心脏微组织显示出存活率的提高,纤维化的发生显着减少。我们的结果表明,妊娠中期人类羊水的剩余样本可以代表 EV 的可行来源,以抵消靶细胞的氧化损伤,从而为抵消骨骼和心肌损伤提供一种新的候选治疗选择。
更新日期:2024-06-17
中文翻译:

来自妊娠中期人类羊水的细胞外囊泡作为旁分泌输送器对抗氧化应激
我们之前证明,妊娠第二个月的人羊水(hAF)是基质祖细胞(人羊水干细胞,hAFSC)的可行来源,具有再生医学的显着旁分泌潜力。从 hAFSC 分泌蛋白组中分离和浓缩的细胞外囊泡 (EV) 可以在骨骼和心肌损伤的临床前模型中发挥促生存、增殖、抗纤维化和心脏保护作用。虽然 hAFSC-EV 分离可能受到体外细胞培养的显着影响,但在这里我们分析了直接从 hAF 浓缩的 EV 作为替代选择,并研究了它们对抗氧化应激的旁分泌潜力。妊娠中期 hAF 样本是在书面知情同意后作为产前诊断羊膜穿刺术的剩余材料获得的。通过尺寸排阻色谱分离 EV,并通过超速离心浓缩。通过纳米颗粒跟踪分析、透射电子显微镜、蛋白质印迹和流式细胞术评估 hAF-EV;通过血氧测定和发光分析评估它们的代谢活性,并通过蛋白质组学和 RNA 测序对它们的货物进行分析。在小鼠 C2C12 细胞和 3D 人类心脏微组织的氧化应激和功能障碍的临床前体外模型中测试了 hAF-EV 旁分泌潜力。我们的方案产生了每 hAF 毫升 6.31 ± 0.98 × 10 EVs 颗粒的产量,显示圆杯形形态和 209.63 ± 6.10 nm 平均尺寸,并具有 CD81、CD63 和 CD9 四跨膜蛋白标记物的相关表达。 hAF-EV 富含 CD133/1、CD326、CD24、CD29 和 SSEA4,并且能够通过耗氧产生 ATP。 虽然氧化应激显着降低了 C2C12 的存活率,但 hAF-EV 引发可显着挽救细胞活力,显着恢复 ATP 合成,同时减少细胞损伤和脂质过氧化活性。用 hAF-EV 处理并经历 H2O2 应激和 TGFβ 刺激的 3D 人类心脏微组织显示出存活率的提高,纤维化的发生显着减少。我们的结果表明,妊娠中期人类羊水的剩余样本可以代表 EV 的可行来源,以抵消靶细胞的氧化损伤,从而为抵消骨骼和心肌损伤提供一种新的候选治疗选择。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号