Clinical Drug Investigation ( IF 2.9 ) Pub Date : 2024-06-21 , DOI: 10.1007/s40261-024-01377-9 Ali Azargoonjahromi 1
|
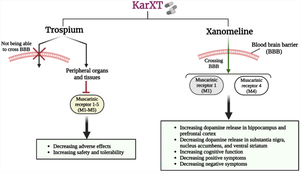
Standard schizophrenia treatment involves antipsychotic medications that target D2 dopamine receptors. However, these drugs have limitations in addressing all symptoms and can lead to adverse effects such as motor impairments, metabolic effects, sedation, sexual dysfunction, cognitive impairment, and tardive dyskinesia. Recently, KarXT has emerged as a novel drug for schizophrenia. KarXT combines xanomeline, a muscarinic receptor M1 and M4 agonist, with trospium, a nonselective antimuscarinic agent. Of note, xanomeline can readily cross blood–brain barrier (BBB) and, thus, enter into the brain, thereby stimulating muscarinic receptors (M1 and M4). By doing so, xanomeline has been shown to target negative symptoms and potentially improve positive symptoms. Trospium, on the other hand, is not able to cross BBB, thereby not affecting M1 and M4 receptors; instead, it acts as an antimuscarinic agent and, hence, diminishes peripheral activity of muscarinic receptors to minimize side effects probably stemming from xanomeline in other organs. Accordingly, ongoing clinical trials investigating KarXT’s efficacy in schizophrenia have demonstrated positive outcomes, including significant improvements in the Positive and Negative Syndrome Scale (PANSS) total score and cognitive function compared with placebo. These findings emphasize the potential of KarXT as a promising treatment for schizophrenia, providing symptom relief while minimizing side effects associated with xanomeline monotherapy. Despite such promising evidence, further research is needed to confirm the efficacy, safety, and tolerability of KarXT in managing schizophrenia. This review article explores the current findings and potential mechanisms of KarXT in the treatment of schizophrenia.
Graphical Abstract
KarXT, a promising medication for schizophrenia, combines xanomeline, an agonist for muscarinic receptors M1 and M4, with trospium, an antimuscarinic agent. Xanomeline can effectively penetrate the blood–brain barrier (BBB), allowing it to target and stimulate muscarinic receptors in the brain, leading to decline or eliminate of positive and negative symptoms as well as cognitive improvement. On the other hand, trospium lacks the ability to cross the BBB and primarily acts as an antimuscarinic agent in peripheral organs, reducing the potential side effects associated with xanomeline. These findings underscore the potential of KarXT as a viable treatment option for schizophrenia, offering symptom relief while minimizing the adverse effects typically associated with xanomeline as a monotherapy.
中文翻译:

KarXT(Xanomeline-Trospium)治疗精神分裂症的最新发现和潜在机制
标准精神分裂症治疗涉及针对 D2 多巴胺受体的抗精神病药物。然而,这些药物在解决所有症状方面存在局限性,并且可能导致不良反应,例如运动障碍、代谢影响、镇静、性功能障碍、认知障碍和迟发性运动障碍。最近,KarXT 成为一种治疗精神分裂症的新药。 KarXT 结合了 xanomeline(一种毒蕈碱受体 M1 和 M4 激动剂)与曲司氯铵(一种非选择性抗毒蕈碱剂)。值得注意的是,xanomeline 很容易穿过血脑屏障 (BBB),从而进入大脑,从而刺激毒蕈碱受体(M1 和 M4)。通过这样做,xanomeline 已被证明可以针对阴性症状并可能改善阳性症状。另一方面,Trospium 不能穿过 BBB,因此不会影响 M1 和 M4 受体;相反,它充当抗毒蕈碱剂,因此减少毒蕈碱受体的外周活性,以最大限度地减少可能源自其他器官的xanomeline的副作用。因此,正在进行的研究 KarXT 对精神分裂症疗效的临床试验已经证明了积极的结果,包括与安慰剂相比,阳性和阴性综合症量表 (PANSS) 总分和认知功能的显着改善。这些发现强调了 KarXT 作为一种有前途的精神分裂症治疗方法的潜力,可以缓解症状,同时最大限度地减少与 xanomeline 单一疗法相关的副作用。尽管有这些有希望的证据,但仍需要进一步的研究来证实 KarXT 在治疗精神分裂症方面的有效性、安全性和耐受性。这篇综述文章探讨了 KarXT 治疗精神分裂症的最新发现和潜在机制。
图解摘要
KarXT 是一种很有前途的治疗精神分裂症的药物,它结合了 xanomeline(一种毒蕈碱受体 M1 和 M4 的激动剂)和曲司氯铵(一种抗毒蕈碱剂)。 Xanomeline 可以有效穿透血脑屏障 (BBB),使其靶向并刺激大脑中的毒蕈碱受体,从而减少或消除阳性和阴性症状以及认知改善。另一方面,曲司氯胺缺乏穿过血脑屏障的能力,主要在外周器官中充当抗毒蕈碱剂,减少与xanomeline相关的潜在副作用。这些发现强调了 KarXT 作为精神分裂症可行治疗选择的潜力,可缓解症状,同时最大限度地减少通常与 xanomeline 作为单一疗法相关的不良反应。













































 京公网安备 11010802027423号
京公网安备 11010802027423号