Cell Stem Cell ( IF 19.8 ) Pub Date : 2024-06-21 , DOI: 10.1016/j.stem.2024.05.011 Shira Landau 1 , Yimu Zhao 1 , Homaira Hamidzada 2 , Gregory M Kent 3 , Sargol Okhovatian 1 , Rick Xing Ze Lu 1 , Chuan Liu 1 , Karl T Wagner 4 , Krisco Cheung 5 , Sarah A Shawky 6 , Daniel Vosoughi 7 , Erika Leigh Beroncal 8 , Ian Fernandes 3 , Carolyn L Cummins 9 , Ana C Andreazza 10 , Gordon M Keller 3 , Slava Epelman 11 , Milica Radisic 12
|
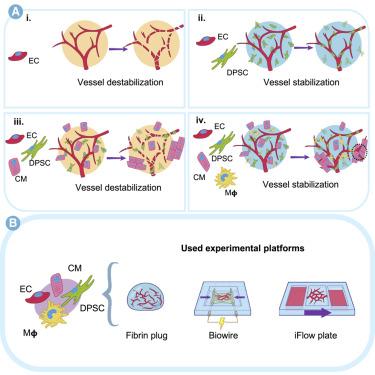
The intricate anatomical structure and high cellular density of the myocardium complicate the bioengineering of perfusable vascular networks within cardiac tissues. In vivo neonatal studies highlight the key role of resident cardiac macrophages in post-injury regeneration and angiogenesis. Here, we integrate human pluripotent stem-cell-derived primitive yolk-sac-like macrophages within vascularized heart-on-chip platforms. Macrophage incorporation profoundly impacted the functionality and perfusability of microvascularized cardiac tissues up to 2 weeks of culture. Macrophages mitigated tissue cytotoxicity and the release of cell-free mitochondrial DNA (mtDNA), while upregulating the secretion of pro-angiogenic, matrix remodeling, and cardioprotective cytokines. Bulk RNA sequencing (RNA-seq) revealed an upregulation of cardiac maturation and angiogenesis genes. Further, single-nuclei RNA sequencing (snRNA-seq) and secretome data suggest that macrophages may prime stromal cells for vascular development by inducing insulin like growth factor binding protein 7 (IGFBP7) and hepatocyte growth factor (HGF) expression. Our results underscore the vital role of primitive macrophages in the long-term vascularization of cardiac tissues, offering insights for therapy and advancing heart-on-a-chip technologies.
中文翻译:

原始巨噬细胞能够实现人类心脏芯片平台的长期血管化
心肌复杂的解剖结构和高细胞密度使心脏组织内可灌注血管网络的生物工程变得复杂。体内新生儿研究强调了常驻心脏巨噬细胞在损伤后再生和血管生成中的关键作用。在这里,我们将人类多能干细胞衍生的原始卵黄囊样巨噬细胞整合到血管化心脏芯片平台中。在培养长达两周的时间里,巨噬细胞的掺入深刻影响了微血管化心脏组织的功能和灌注性。巨噬细胞减轻组织细胞毒性和无细胞线粒体 DNA (mtDNA) 的释放,同时上调促血管生成、基质重塑和心脏保护细胞因子的分泌。大量 RNA 测序 (RNA-seq) 揭示了心脏成熟和血管生成基因的上调。此外,单核 RNA 测序 (snRNA-seq) 和分泌组数据表明,巨噬细胞可能通过诱导胰岛素样生长因子结合蛋白 7 (IGFBP7) 和肝细胞生长因子 (HGF) 的表达,为血管发育启动基质细胞。我们的结果强调了原始巨噬细胞在心脏组织长期血管化中的重要作用,为治疗和推进芯片心脏技术提供了见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号