当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rationally Designed Confined Structure Ce-Mn-TNTs Catalyst for Low-Temperature NH3-SCR Reaction with Superior Activity and H2O/SO2 Tolerance
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-06-20 , DOI: 10.1021/acssuschemeng.4c03170 Qiang Zhao 1, 2 , Xiaosheng Huang 2, 3 , Tian Zhao 2 , Rongji Cui 2, 3 , Jiyi Zhang 1 , Zhicheng Tang 2, 3
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-06-20 , DOI: 10.1021/acssuschemeng.4c03170 Qiang Zhao 1, 2 , Xiaosheng Huang 2, 3 , Tian Zhao 2 , Rongji Cui 2, 3 , Jiyi Zhang 1 , Zhicheng Tang 2, 3
Affiliation
|
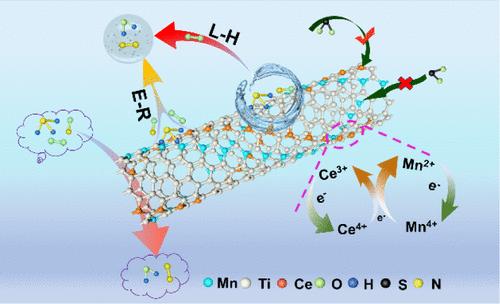
The SO2 tolerance of catalysts is often the key factor limiting their catalytic activity during practical applications for low-temperature NH3-SCR reaction. There has been no in-depth research and exploration on the confined location of active components so far. In this work, we investigated the activity and SO2 tolerance of catalysts by loading Mn into cerium-titanium nanotubes (Ce-TNTs) in three different ways. It was found that the Ce-Mn-TNT catalyst with Mn confined in a nanotube structure by the direct strong alkali hydrothermal method showed excellent catalytic activity and strong resistance to SO2 and H2O. This was mainly due to the unique nanotube structure, which provided a large specific surface area and promoted uniform dispersion of active sites. Importantly, the confinement effect of the nanotube structure accelerated the electron transfer rate among Ce, Mn, and Ti metals, which enhanced the redox performance of the catalyst and improved the resistance to SO2. In addition, it was also found that the introduction of Mn increased the amounts of weak acid sites and inhibited oxygen inhibition, enhanced the adsorption of NO on the catalyst surface, and formed more lattice defect structures. Finally, the possible reaction mechanism of the Ce-Mn-TNT catalyst was investigated by in situ DRIFTs. When the gas reaction temperature was 200 °C, it was found that the E-R and L-H reaction pathways coexisted in the NH3-SCR reaction when SO2 was present in the reaction atmosphere.
中文翻译:

合理设计的限域结构 Ce-Mn-TNT 催化剂用于低温 NH3-SCR 反应,具有优异的活性和 H2O/SO2 耐受性
在低温NH 3 -SCR反应实际应用中,催化剂的SO 2 耐受性往往是限制其催化活性的关键因素。目前尚未对有源部件的受限位置进行深入的研究和探索。在这项工作中,我们通过三种不同的方式将锰负载到铈钛纳米管(Ce-TNT)中,研究了催化剂的活性和 SO 2 耐受性。结果发现,采用直接强碱水热法将Mn限制在纳米管结构中的Ce-Mn-TNT催化剂表现出优异的催化活性,并对SO 2 和H 2 具有较强的抵抗能力。这主要是由于独特的纳米管结构,提供了很大的比表面积,促进了活性位点的均匀分散。重要的是,纳米管结构的限域效应加速了Ce、Mn和Ti金属之间的电子转移速率,从而增强了催化剂的氧化还原性能并提高了对SO 2 的抵抗能力。此外,还发现Mn的引入增加了弱酸位的数量并抑制了氧抑制,增强了NO在催化剂表面的吸附,并形成了更多的晶格缺陷结构。最后,通过原位 DRIFT 研究了 Ce-Mn-TNT 催化剂可能的反应机理。当气体反应温度为200 ℃时,发现当反应气氛中存在SO 2 时,NH 3 -SCR反应中E-R和L-H反应途径共存。
更新日期:2024-06-20
中文翻译:

合理设计的限域结构 Ce-Mn-TNT 催化剂用于低温 NH3-SCR 反应,具有优异的活性和 H2O/SO2 耐受性
在低温NH 3 -SCR反应实际应用中,催化剂的SO 2 耐受性往往是限制其催化活性的关键因素。目前尚未对有源部件的受限位置进行深入的研究和探索。在这项工作中,我们通过三种不同的方式将锰负载到铈钛纳米管(Ce-TNT)中,研究了催化剂的活性和 SO 2 耐受性。结果发现,采用直接强碱水热法将Mn限制在纳米管结构中的Ce-Mn-TNT催化剂表现出优异的催化活性,并对SO 2 和H 2 具有较强的抵抗能力。这主要是由于独特的纳米管结构,提供了很大的比表面积,促进了活性位点的均匀分散。重要的是,纳米管结构的限域效应加速了Ce、Mn和Ti金属之间的电子转移速率,从而增强了催化剂的氧化还原性能并提高了对SO 2 的抵抗能力。此外,还发现Mn的引入增加了弱酸位的数量并抑制了氧抑制,增强了NO在催化剂表面的吸附,并形成了更多的晶格缺陷结构。最后,通过原位 DRIFT 研究了 Ce-Mn-TNT 催化剂可能的反应机理。当气体反应温度为200 ℃时,发现当反应气氛中存在SO 2 时,NH 3 -SCR反应中E-R和L-H反应途径共存。











































 京公网安备 11010802027423号
京公网安备 11010802027423号