当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhancing V2O5 Cathode Performance through Heterostructure Engineering with the Ti3C2O2 MXene: A Computational Study
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-06-20 , DOI: 10.1021/acs.jpcc.3c08078 Pariwut Falun 1 , Lappawat Ngamwongwan 2 , Sirisak Singsen 2 , Maneerat Chotsawat 1 , Paratee Komen 1 , Anchalee Junkaew 3 , Suwit Suthirakun 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-06-20 , DOI: 10.1021/acs.jpcc.3c08078 Pariwut Falun 1 , Lappawat Ngamwongwan 2 , Sirisak Singsen 2 , Maneerat Chotsawat 1 , Paratee Komen 1 , Anchalee Junkaew 3 , Suwit Suthirakun 1
Affiliation
|
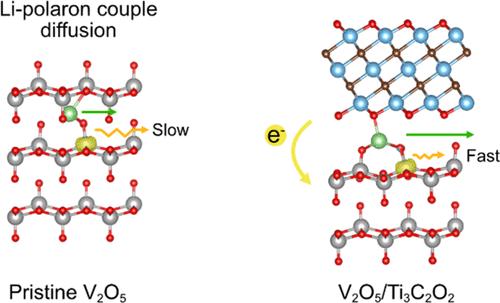
V2O5 has been proposed as a potential candidate for cathode materials of Li-ion batteries due to its high theoretical capacity and cost effectiveness, but it still suffers from high capacity fading and slow charge/discharge kinetics. To improve its electrochemical performance, heterostructure engineering with the Ti3C2O2 MXene was computationally studied in this work. Herein, we carried out density functional theory calculations to study such effects on the electronic conductivity and Li intercalation kinetics of the cathode. We find that the formation of V2O5/Ti3C2O2 is energetically favorable where the interaction at the interface is characterized as a weak van der Waals force. When the heterostructure is formed, electrons are transferred from Ti3C2O2 to the V2O5 surface where the charge accumulation induces small lattice distortion of the inner V2O5 layer. Such charge accumulation and distortions, in turn, reduce the polaron–lattice interaction, leading to less stable polaron formation energy when compared with that of bulk V2O5 (−0.20 vs −0.35 eV). This weakened polaron–lattice interaction enhances the polaron hopping kinetics as it correlates with smaller polaron hopping barriers. The higher hopping rate constant of the polaron alleviates the Li intercalation kinetics where the ion-coupled polaron movement, used to have polaron hopping as rate-limiting, is now ion diffusion-limiting with somewhat smaller barriers. The calculated average diffusion rate constant is slightly higher at the heterostructure (4.03 × 108 s–1) than that in bulk V2O5 (2.17 × 108 s–1). Overall, it is suggested by our computational study that the improved electronic conductivity and ion diffusion kinetics could have their origin from the enhanced rate constant of polaron hopping at the interface of the heterostructure.
中文翻译:

通过 Ti3C2O2 MXene 异质结构工程提高 V2O5 阴极性能:计算研究
V 2 O 5 由于其高理论容量和成本效益而被提议作为锂离子电池正极材料的潜在候选材料,但它仍然存在高容量衰减和高容量损耗的问题。缓慢的充电/放电动力学。为了提高其电化学性能,本工作对 Ti 3 C 2 O 2 MXene 的异质结构工程进行了计算研究。在这里,我们进行了密度泛函理论计算,以研究这种对阴极电子电导率和锂嵌入动力学的影响。我们发现 V 2 O 5 /Ti 3 C 2 O 2 的形成在能量上是有利的其中界面处的相互作用被表征为弱范德华力。当异质结构形成时,电子从 Ti 3 C 2 O 2 转移到 V 2 O 5 O 5 层的小晶格畸变。这种电荷积累和扭曲反过来会减少极化子-晶格相互作用,导致与体相 V 2 O 5 相比不稳定的极化子形成能 (−0.20 vs − 0.35 eV)。这种减弱的极化子-晶格相互作用增强了极化子跳跃动力学,因为它与较小的极化子跳跃势垒相关。极化子的较高跳跃速率常数减轻了锂嵌入动力学,其中离子耦合极化子运动过去以极化子跳跃作为速率限制,现在以较小的势垒限制离子扩散。计算得出的异质结构平均扩散速率常数略高 (4.03 × 10 8 s –1 ) 比散装 V 2 O 5 (2.17 × 10 8 –1 )。总的来说,我们的计算研究表明,改进的电子电导率和离子扩散动力学可能源于异质结构界面处极化子跳跃速率常数的增强。
更新日期:2024-06-21
中文翻译:

通过 Ti3C2O2 MXene 异质结构工程提高 V2O5 阴极性能:计算研究
V 2 O 5 由于其高理论容量和成本效益而被提议作为锂离子电池正极材料的潜在候选材料,但它仍然存在高容量衰减和高容量损耗的问题。缓慢的充电/放电动力学。为了提高其电化学性能,本工作对 Ti 3 C 2 O 2 MXene 的异质结构工程进行了计算研究。在这里,我们进行了密度泛函理论计算,以研究这种对阴极电子电导率和锂嵌入动力学的影响。我们发现 V 2 O 5 /Ti 3 C 2 O 2 的形成在能量上是有利的其中界面处的相互作用被表征为弱范德华力。当异质结构形成时,电子从 Ti 3 C 2 O 2 转移到 V 2 O 5 O 5 层的小晶格畸变。这种电荷积累和扭曲反过来会减少极化子-晶格相互作用,导致与体相 V 2 O 5 相比不稳定的极化子形成能 (−0.20 vs − 0.35 eV)。这种减弱的极化子-晶格相互作用增强了极化子跳跃动力学,因为它与较小的极化子跳跃势垒相关。极化子的较高跳跃速率常数减轻了锂嵌入动力学,其中离子耦合极化子运动过去以极化子跳跃作为速率限制,现在以较小的势垒限制离子扩散。计算得出的异质结构平均扩散速率常数略高 (4.03 × 10 8 s –1 ) 比散装 V 2 O 5 (2.17 × 10 8 –1 )。总的来说,我们的计算研究表明,改进的电子电导率和离子扩散动力学可能源于异质结构界面处极化子跳跃速率常数的增强。






































 京公网安备 11010802027423号
京公网安备 11010802027423号