当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Uncovering the Influence Mechanism of Solvent on Crystal Morphology via a Combined Experimental and Theoretical Method: The Case of Aceclofenac
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-06-18 , DOI: 10.1021/acs.iecr.4c01283 Longping Jiang 1 , Mengmeng Wang 2 , Wenqian Chen 3 , Limin Zhou 1 , Li Xu 1 , Hamza Shehzad 1 , Jinbo Ouyang 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-06-18 , DOI: 10.1021/acs.iecr.4c01283 Longping Jiang 1 , Mengmeng Wang 2 , Wenqian Chen 3 , Limin Zhou 1 , Li Xu 1 , Hamza Shehzad 1 , Jinbo Ouyang 1
Affiliation
|
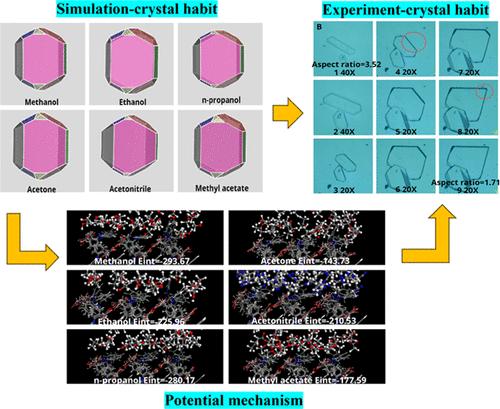
Aceclofenac (ACF) is a kind of antipyretic, analgesic, and antiarthritic drug, which is clinically suitable for the treatment of rheumatoid arthritis. However, the morphology of ACF produced in the crystallization process is easily regulated by different solvents, which has a significant effect on the powder properties of the ACF crystals. Therefore, the study of crystal habits is needed for producing ACF with a better performance. In this study, the effects of different solvents on the growth of the ACF crystal were studied by analyzing the morphology of ACF in various solvents and comprehensive simulation calculations. It was found that the ACF crystals in ethanol (EAL) and acetonitrile (ACE) were quadrangular in shape. In methyl acetate (MA), acetone (ACT), and n-propanol (NPL), the lateral aspect of the (101̅) facet is clearly prominent. More interestingly, pentagonal crystals are found only in methanol (MEL), and the (002) facet eventually disappears as they grow. Through molecular dynamics simulations, we have found that the attachment energy Eatt of crystal facets, the Connolly surface coefficient R, and solvent effects Es are the main factors influencing the growth of ACF crystals. The competitive adsorption of ACF and solvent molecules onto crystal facets accounts for morphology change during the growth process. By establishing a strong link between experimental observations and simulation results, this study favors the morphology control of the ACF crystal.
中文翻译:

实验与理论相结合揭示溶剂对晶体形貌的影响机制——以醋氯芬酸为例
醋氯芬酸(ACF)是一种解热、镇痛、抗关节炎药物,临床适用于治疗类风湿性关节炎。然而,结晶过程中产生的ACF形貌很容易受到不同溶剂的调控,这对ACF晶体的粉末性能有显着影响。因此,需要对晶体习性进行研究,以生产出性能更好的ACF。本研究通过分析ACF在各种溶剂中的形貌并综合模拟计算,研究了不同溶剂对ACF晶体生长的影响。结果发现乙醇(EAL)和乙腈(ACE)中的ACF晶体呈四边形。在乙酸甲酯 (MA)、丙酮 (ACT) 和正丙醇 (NPL) 中,(101̅) 面的侧面明显突出。更有趣的是,五方晶体仅存在于甲醇(MEL)中,并且(002)晶面最终随着它们的生长而消失。通过分子动力学模拟,我们发现晶面附着能E att 、康诺利表面系数R和溶剂效应E s 是影响ACF生长的主要因素晶体。 ACF 和溶剂分子在晶体面上的竞争吸附导致了生长过程中的形态变化。通过在实验观察和模拟结果之间建立强有力的联系,本研究有利于 ACF 晶体的形貌控制。
更新日期:2024-06-18
中文翻译:

实验与理论相结合揭示溶剂对晶体形貌的影响机制——以醋氯芬酸为例
醋氯芬酸(ACF)是一种解热、镇痛、抗关节炎药物,临床适用于治疗类风湿性关节炎。然而,结晶过程中产生的ACF形貌很容易受到不同溶剂的调控,这对ACF晶体的粉末性能有显着影响。因此,需要对晶体习性进行研究,以生产出性能更好的ACF。本研究通过分析ACF在各种溶剂中的形貌并综合模拟计算,研究了不同溶剂对ACF晶体生长的影响。结果发现乙醇(EAL)和乙腈(ACE)中的ACF晶体呈四边形。在乙酸甲酯 (MA)、丙酮 (ACT) 和正丙醇 (NPL) 中,(101̅) 面的侧面明显突出。更有趣的是,五方晶体仅存在于甲醇(MEL)中,并且(002)晶面最终随着它们的生长而消失。通过分子动力学模拟,我们发现晶面附着能E att 、康诺利表面系数R和溶剂效应E s 是影响ACF生长的主要因素晶体。 ACF 和溶剂分子在晶体面上的竞争吸附导致了生长过程中的形态变化。通过在实验观察和模拟结果之间建立强有力的联系,本研究有利于 ACF 晶体的形貌控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号