当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
pH Effects on Electrocatalytic H2O2 Production and Flooding in Gas-Diffusion Electrodes for Oxygen Reduction Reaction
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-06-19 , DOI: 10.1021/acssuschemeng.4c02325 Takuya Okazaki 1 , Kento Shibata 2 , Chihiro Tateishi 2 , Kazuma Enomoto 1 , Kosuke Beppu 1 , Fumiaki Amano 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-06-19 , DOI: 10.1021/acssuschemeng.4c02325 Takuya Okazaki 1 , Kento Shibata 2 , Chihiro Tateishi 2 , Kazuma Enomoto 1 , Kosuke Beppu 1 , Fumiaki Amano 1
Affiliation
|
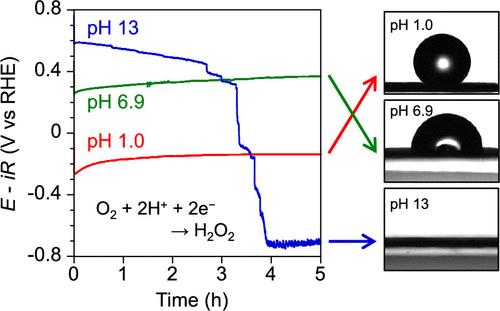
Electrocatalytic two-electron oxygen reduction reaction (2e– ORR) is a crucial process for on-site and on-demand H2O2 production. Evaluating the impact of the medium’s pH is essential for achieving selective H2O2 production at higher current density through continuous O2 supply to gas diffusion electrodes (GDEs) in membrane electrolyzers. We investigated the effect of electrolyte pH on both H2O2 production and the flooding behavior of GDEs loaded with a cobalt single-atom catalyst in a typical H-type cell. The electrocatalyst was prepared by heating cobalt(II) tetraphenylporphyrin loaded on Ketjen Black (CoTPP/KB) at 750 °C. Remarkably, the cobalt single-atom catalyst exhibited high current density when the top of the hydrophobic gas-diffusion layer was exposed to the gas phase, facilitating efficient O2 diffusion within the GDE. Potential–time curves at −40 mA cm–2 showed stable potentials and Faradaic efficiencies (>80%) over 5 h across a pH range from 1 to 10, with the H2O2 concentration reaching approximately 100 mmol L–1 at pH 1.0. In contrast, the potential at pH 13 decreased abruptly in 3 h due to the flooding of GDE. Long-term tests demonstrated stable electrocatalytic H2O2 production only in the acidic electrolytes for 24 h, attributed to reduced flooding with decreasing pH. These findings underscore the impact of electrolyte pH on GDE performance during H2O2 production via the 2e– ORR, with acidity favoring the mitigation of undesirable flooding behavior.
中文翻译:

pH 对氧还原反应气体扩散电极中电催化 H2O2 生成和溢流的影响
电催化双电子氧还原反应(2e – ORR)是现场按需生产 H 2 O 2 的关键过程。评估介质 pH 值的影响对于通过向气体扩散电极 (GDE) 连续供应 O 2 在较高电流密度下实现选择性 H 2 O 2 生产至关重要)在膜电解槽中。我们研究了电解质 pH 值对 H 2 O 2 产生的影响以及在典型 H 型电池中负载钴单原子催化剂的 GDE 的驱替行为。该电催化剂是通过在 750 °C 下加热负载在科琴黑 (CoTPP/KB) 上的四苯基卟啉钴 (II) 来制备的。值得注意的是,当疏水性气体扩散层的顶部暴露于气相时,钴单原子催化剂表现出高电流密度,促进 O 2 在 GDE 内有效扩散。 -40 mA cm –2 下的电位-时间曲线显示出在 1 至 10 的 pH 范围内 5 小时内稳定的电位和法拉第效率 (>80%),其中 H 2 O 2 浓度在 pH 1.0 时达到约 100 mmol L –1 。相反,由于 GDE 的淹没,pH 13 时的电位在 3 小时内突然下降。长期测试表明,电催化 H 2 O 2 仅在酸性电解质中稳定生产 24 小时,这归因于随着 pH 值降低而减少了水淹。这些发现强调了通过 2e – ORR 生产 H 2 O 2 过程中电解质 pH 值对 GDE 性能的影响,酸度有利于减轻不良的溢流行为。
更新日期:2024-06-20
中文翻译:

pH 对氧还原反应气体扩散电极中电催化 H2O2 生成和溢流的影响
电催化双电子氧还原反应(2e – ORR)是现场按需生产 H 2 O 2 的关键过程。评估介质 pH 值的影响对于通过向气体扩散电极 (GDE) 连续供应 O 2 在较高电流密度下实现选择性 H 2 O 2 生产至关重要)在膜电解槽中。我们研究了电解质 pH 值对 H 2 O 2 产生的影响以及在典型 H 型电池中负载钴单原子催化剂的 GDE 的驱替行为。该电催化剂是通过在 750 °C 下加热负载在科琴黑 (CoTPP/KB) 上的四苯基卟啉钴 (II) 来制备的。值得注意的是,当疏水性气体扩散层的顶部暴露于气相时,钴单原子催化剂表现出高电流密度,促进 O 2 在 GDE 内有效扩散。 -40 mA cm –2 下的电位-时间曲线显示出在 1 至 10 的 pH 范围内 5 小时内稳定的电位和法拉第效率 (>80%),其中 H 2 O 2 浓度在 pH 1.0 时达到约 100 mmol L –1 。相反,由于 GDE 的淹没,pH 13 时的电位在 3 小时内突然下降。长期测试表明,电催化 H 2 O 2 仅在酸性电解质中稳定生产 24 小时,这归因于随着 pH 值降低而减少了水淹。这些发现强调了通过 2e – ORR 生产 H 2 O 2 过程中电解质 pH 值对 GDE 性能的影响,酸度有利于减轻不良的溢流行为。






































 京公网安备 11010802027423号
京公网安备 11010802027423号