Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Programmed Cascade Polydopamine Nanoclusters for Pyroptosis-Based Tumor Immunotherapy
Small ( IF 13.0 ) Pub Date : 2024-06-19 , DOI: 10.1002/smll.202401397
Zeyu Han 1 , Yan Liang 2 , Yan Li 3 , Mujie Yuan 1 , Xin Zhan 1 , Jianqin Yan 2 , Yong Sun 2 , Kui Luo 4 , Baodong Zhao 1 , Fan Li 1, 2
Small ( IF 13.0 ) Pub Date : 2024-06-19 , DOI: 10.1002/smll.202401397
Zeyu Han 1 , Yan Liang 2 , Yan Li 3 , Mujie Yuan 1 , Xin Zhan 1 , Jianqin Yan 2 , Yong Sun 2 , Kui Luo 4 , Baodong Zhao 1 , Fan Li 1, 2
Affiliation
|
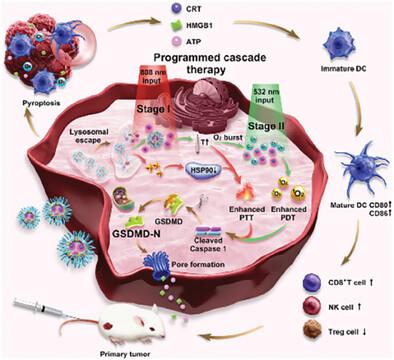
Pyroptosis, an inflammatory cell death, plays a pivotal role in activating inflammatory response, reversing immunosuppression and enhancing anti-tumor immunity. However, challenges remain regarding how to induce pyroptosis efficiently and precisely in tumor cells to amplify anti-tumor immunotherapy. Herein, a pH-responsive polydopamine (PDA) nanocluster, perfluorocarbon (PFC)@octo-arginine (R8)-1-Hexadecylamine (He)-porphyrin (Por)@PDA-gambogic acid (GA)-cRGD (R-P@PDA-GC), is rationally design to augment phototherapy-induced pyroptosis and boost anti-tumor immunity through a two-input programmed cascade therapy. Briefly, oxygen doner PFC is encapsulated within R8 linked photosensitizer Por and He micelles as the core, followed by incorporation of GA and cRGD peptides modified PDA shell, yielding the ultimate R-P@PDA-GC nanoplatforms (NPs). The pH-responsive NPs effectively alleviate hypoxia by delivering oxygen via PFC and mitigate heat resistance in tumor cells through GA. Upon two-input programmed irradiation, R-P@PDA-GC NPs significantly enhance reactive oxygen species production within tumor cells, triggering pyroptosis via the Caspase-1/GSDMD pathway and releasing numerous inflammatory factors into the TME. This leads to the maturation of dendritic cells, robust infiltration of cytotoxic CD8+ T and NK cells, and diminution of immune suppressor Treg cells, thereby amplifying anti-tumor immunity.
中文翻译:

用于基于 Pypyroptis 的肿瘤免疫治疗的程序化级联多巴胺纳米簇
焦亡是一种炎症细胞死亡,在激活炎症反应、逆转免疫抑制和增强抗肿瘤免疫方面起着关键作用。然而,如何在肿瘤细胞中有效、精确地诱导细胞焦亡以放大抗肿瘤免疫治疗仍然存在挑战。在此,合理设计了 pH 响应性聚多巴胺 (PDA) 纳米簇,全氟化碳 (PFC)@octo-精氨酸 (R8)-1-十六烷胺 (He)-卟啉 (Por)@PDA-藤黄酸 (GA)-cRGD (R-P@PDA-GC),通过双输入程序级联疗法增强光疗诱导的焦亡并增强抗肿瘤免疫力。简而言之,氧供体 PFC 被封装在 R8 连接的光敏剂 Por 和 He 胶束中作为核心,然后掺入 GA 和 cRGD 肽修饰的 PDA 壳,产生最终的 R-P@PDA-GC 纳米平台 (NPs)。pH 响应性 NPs 通过 PFC 输送氧气有效缓解缺氧,并通过 GA 减轻肿瘤细胞的热阻。在双输入程序性照射后,R-P@PDA-GC NPs 显着增强肿瘤细胞内活性氧的产生,通过 Caspase-1/GSDMD 通路触发细胞焦亡,并将许多炎症因子释放到 TME 中。这导致树突状细胞成熟,细胞毒性 CD8+ T 和 NK 细胞的强烈浸润,以及免疫抑制性 Treg 细胞的减少,从而增强抗肿瘤免疫力。
更新日期:2024-06-19
中文翻译:

用于基于 Pypyroptis 的肿瘤免疫治疗的程序化级联多巴胺纳米簇
焦亡是一种炎症细胞死亡,在激活炎症反应、逆转免疫抑制和增强抗肿瘤免疫方面起着关键作用。然而,如何在肿瘤细胞中有效、精确地诱导细胞焦亡以放大抗肿瘤免疫治疗仍然存在挑战。在此,合理设计了 pH 响应性聚多巴胺 (PDA) 纳米簇,全氟化碳 (PFC)@octo-精氨酸 (R8)-1-十六烷胺 (He)-卟啉 (Por)@PDA-藤黄酸 (GA)-cRGD (R-P@PDA-GC),通过双输入程序级联疗法增强光疗诱导的焦亡并增强抗肿瘤免疫力。简而言之,氧供体 PFC 被封装在 R8 连接的光敏剂 Por 和 He 胶束中作为核心,然后掺入 GA 和 cRGD 肽修饰的 PDA 壳,产生最终的 R-P@PDA-GC 纳米平台 (NPs)。pH 响应性 NPs 通过 PFC 输送氧气有效缓解缺氧,并通过 GA 减轻肿瘤细胞的热阻。在双输入程序性照射后,R-P@PDA-GC NPs 显着增强肿瘤细胞内活性氧的产生,通过 Caspase-1/GSDMD 通路触发细胞焦亡,并将许多炎症因子释放到 TME 中。这导致树突状细胞成熟,细胞毒性 CD8+ T 和 NK 细胞的强烈浸润,以及免疫抑制性 Treg 细胞的减少,从而增强抗肿瘤免疫力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号