Immunity ( IF 25.5 ) Pub Date : 2024-06-20 , DOI: 10.1016/j.immuni.2024.06.001 Randall T Mertens 1 , Aditya Misra 2 , Peng Xiao 3 , Seungbyn Baek 4 , Joseph M Rone 1 , Davide Mangani 3 , Kisha N Sivanathan 1 , Adedamola S Arojojoye 5 , Samuel G Awuah 6 , Insuk Lee 7 , Guo-Ping Shi 8 , Boryana Petrova 9 , Jeannette R Brook 9 , Ana C Anderson 10 , Richard A Flavell 11 , Naama Kanarek 12 , Martin Hemberg 13 , Roni Nowarski 13
|
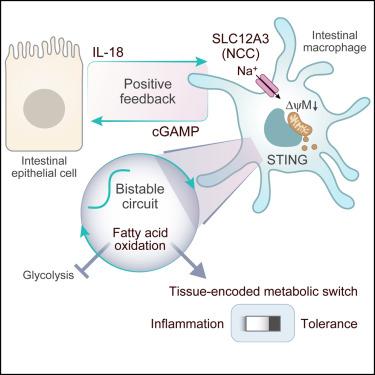
Tissues are exposed to diverse inflammatory challenges that shape future inflammatory responses. While cellular metabolism regulates immune function, how metabolism programs and stabilizes immune states within tissues and tunes susceptibility to inflammation is poorly understood. Here, we describe an innate immune metabolic switch that programs long-term intestinal tolerance. Intestinal interleukin-18 (IL-18) stimulation elicited tolerogenic macrophages by preventing their proinflammatory glycolytic polarization via metabolic reprogramming to fatty acid oxidation (FAO). FAO reprogramming was triggered by IL-18 activation of SLC12A3 (NCC), leading to sodium influx, release of mitochondrial DNA, and activation of stimulator of interferon genes (STING). FAO was maintained in macrophages by a bistable switch that encoded memory of IL-18 stimulation and by intercellular positive feedback that sustained the production of macrophage-derived 2′3′-cyclic GMP–AMP (cGAMP) and epithelial-derived IL-18. Thus, a tissue-reinforced metabolic switch encodes durable immune tolerance in the gut and may enable reconstructing compromised immune tolerance in chronic inflammation.
中文翻译:

IL-18 和环状二核苷酸 cGAMP 协调的代谢开关可调节肠道耐受性
组织面临着多种炎症挑战,这些挑战塑造了未来的炎症反应。虽然细胞代谢调节免疫功能,但代谢如何编程和稳定组织内的免疫状态以及调节炎症的易感性却知之甚少。在这里,我们描述了一种可调节长期肠道耐受性的先天免疫代谢开关。肠道白细胞介素 18 (IL-18) 刺激通过代谢重编程为脂肪酸氧化 (FAO) 来阻止促炎性糖酵解极化,从而引发耐受性巨噬细胞。 FAO 重编程是由 IL-18 激活 SLC12A3 (NCC) 触发的,导致钠流入、线粒体 DNA 释放以及干扰素基因刺激物 (STING) 的激活。巨噬细胞中的FAO通过编码IL-18刺激记忆的双稳态开关和维持巨噬细胞源性2'3'-环GMP-AMP (cGAMP)和上皮源性IL-18产生的细胞间正反馈来维持。因此,组织强化的代谢开关编码肠道中持久的免疫耐受性,并且可能能够重建慢性炎症中受损的免疫耐受性。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号