当前位置:
X-MOL 学术
›
Cancer Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Neoadjuvant SHR-1701 with or without chemotherapy in unresectable stage III non-small-cell lung cancer: A proof-of-concept, phase 2 trial
Cancer Cell ( IF 48.8 ) Pub Date : 2024-06-20 , DOI: 10.1016/j.ccell.2024.05.024 Qing Zhou 1 , Yi Pan 1 , Xuening Yang 1 , Yanqiu Zhao 2 , Guang Han 3 , Qingsong Pang 4 , Zhenfa Zhang 5 , Qifeng Wang 6 , Jun Yao 7 , Hui Wang 8 , Weihua Yang 9 , Baogang Liu 10 , Qixun Chen 11 , Xianghui Du 12 , Kaican Cai 13 , Baosheng Li 14 , Yunchao Huang 15 , Xiao Li 16 , Li Song 16 , Wei Shi 16 , Yi-Long Wu 1
Cancer Cell ( IF 48.8 ) Pub Date : 2024-06-20 , DOI: 10.1016/j.ccell.2024.05.024 Qing Zhou 1 , Yi Pan 1 , Xuening Yang 1 , Yanqiu Zhao 2 , Guang Han 3 , Qingsong Pang 4 , Zhenfa Zhang 5 , Qifeng Wang 6 , Jun Yao 7 , Hui Wang 8 , Weihua Yang 9 , Baogang Liu 10 , Qixun Chen 11 , Xianghui Du 12 , Kaican Cai 13 , Baosheng Li 14 , Yunchao Huang 15 , Xiao Li 16 , Li Song 16 , Wei Shi 16 , Yi-Long Wu 1
Affiliation
|
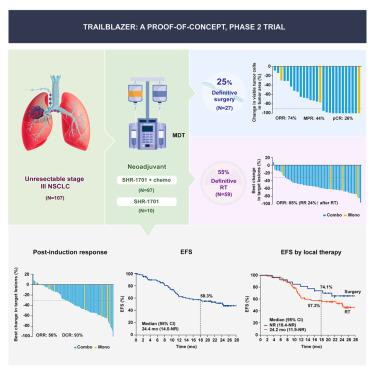
We conducted a proof-of-concept, phase 2 trial to assess neoadjuvant SHR-1701 with or without chemotherapy, followed by surgery or radiotherapy, and then consolidation SHR-1701 in unresectable stage III non-small-cell lung cancer (NSCLC). In the primary cohort of patients receiving neoadjuvant combination therapy ( = 97), both primary endpoints were met, with a post-induction objective response rate of 58% (95% confidence interval [CI] 47–68) and an 18-month event-free survival (EFS) rate of 56.6% (95% CI 45.2–66.5). Overall, 27 (25%) patients underwent surgery; all achieved R0 resection. Among them, 12 (44%) major pathological responses and seven (26%) pathological complete responses were recorded. The 18-month EFS rate was 74.1% (95% CI 53.2–86.7) in surgical patients and 57.3% (43.0–69.3) in radiotherapy-treated patients. Neoadjuvant SHR-1701 with chemotherapy, followed by surgery or radiotherapy, showed promising efficacy with a tolerable safety profile in unresectable stage III NSCLC. Surgical conversion was feasible in a notable proportion of patients and associated with better survival outcomes.
中文翻译:

新辅助 SHR-1701 联合或不联合化疗治疗不可切除的 III 期非小细胞肺癌:概念验证 2 期试验
我们进行了一项概念验证、2 期试验,以评估新辅助 SHR-1701 联合或不联合化疗、随后进行手术或放疗,然后巩固 SHR-1701 治疗不可切除的 III 期非小细胞肺癌 (NSCLC)。在接受新辅助联合治疗的主要患者队列中 (= 97),两个主要终点均得到满足,诱导后客观缓解率为 58%(95% 置信区间 [CI] 47-68),事件发生时间为 18 个月- 无生存率 (EFS) 为 56.6% (95% CI 45.2–66.5)。总体而言,27 名 (25%) 患者接受了手术;全部实现R0切除。其中,记录了 12 例(44%)主要病理反应和 7 例(26%)病理完全反应。手术患者的 18 个月 EFS 率为 74.1% (95% CI 53.2–86.7),而放疗患者的 18 个月 EFS 率为 57.3% (43.0–69.3)。新辅助 SHR-1701 联合化疗,随后进行手术或放疗,在不可切除的 III 期 NSCLC 中显示出良好的疗效和可耐受的安全性。手术转换对于相当大比例的患者是可行的,并且与更好的生存结果相关。
更新日期:2024-06-20
中文翻译:

新辅助 SHR-1701 联合或不联合化疗治疗不可切除的 III 期非小细胞肺癌:概念验证 2 期试验
我们进行了一项概念验证、2 期试验,以评估新辅助 SHR-1701 联合或不联合化疗、随后进行手术或放疗,然后巩固 SHR-1701 治疗不可切除的 III 期非小细胞肺癌 (NSCLC)。在接受新辅助联合治疗的主要患者队列中 (= 97),两个主要终点均得到满足,诱导后客观缓解率为 58%(95% 置信区间 [CI] 47-68),事件发生时间为 18 个月- 无生存率 (EFS) 为 56.6% (95% CI 45.2–66.5)。总体而言,27 名 (25%) 患者接受了手术;全部实现R0切除。其中,记录了 12 例(44%)主要病理反应和 7 例(26%)病理完全反应。手术患者的 18 个月 EFS 率为 74.1% (95% CI 53.2–86.7),而放疗患者的 18 个月 EFS 率为 57.3% (43.0–69.3)。新辅助 SHR-1701 联合化疗,随后进行手术或放疗,在不可切除的 III 期 NSCLC 中显示出良好的疗效和可耐受的安全性。手术转换对于相当大比例的患者是可行的,并且与更好的生存结果相关。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号