Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hierarchical Protofilament Intertwining Rules the Formation of Mixed-Curvature Amyloid Polymorphs
Advanced Science ( IF 14.3 ) Pub Date : 2024-06-20 , DOI: 10.1002/advs.202402740 Jiangtao Zhou 1, 2 , Salvatore Assenza 3, 4, 5 , Meltem Tatli 6 , Jiawen Tian 6 , Ioana M Ilie 7, 8 , Eugene L Starostin 9 , Amedeo Caflisch 10 , Tuomas P J Knowles 11 , Giovanni Dietler 1 , Francesco S Ruggeri 12, 13 , Henning Stahlberg 6 , Sergey K Sekatskii 1, 6 , Raffaele Mezzenga 2, 14
Advanced Science ( IF 14.3 ) Pub Date : 2024-06-20 , DOI: 10.1002/advs.202402740 Jiangtao Zhou 1, 2 , Salvatore Assenza 3, 4, 5 , Meltem Tatli 6 , Jiawen Tian 6 , Ioana M Ilie 7, 8 , Eugene L Starostin 9 , Amedeo Caflisch 10 , Tuomas P J Knowles 11 , Giovanni Dietler 1 , Francesco S Ruggeri 12, 13 , Henning Stahlberg 6 , Sergey K Sekatskii 1, 6 , Raffaele Mezzenga 2, 14
Affiliation
|
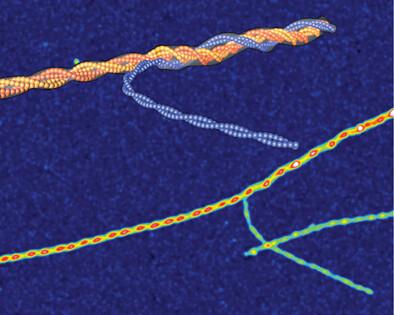
Amyloid polymorphism is a hallmark of almost all amyloid species, yet the mechanisms underlying the formation of amyloid polymorphs and their complex architectures remain elusive. Commonly, two main mesoscopic topologies are found in amyloid polymorphs characterized by non-zero Gaussian and mean curvatures: twisted ribbons and helical fibrils, respectively. Here, a rich heterogeneity of configurations is demonstrated on insulin amyloid fibrils, where protofilament packing can occur, besides the common polymorphs, also in a combined mode forming mixed-curvature polymorphs. Through AFM statistical analysis, an extended array of heterogeneous architectures that are rationalized by mesoscopic theoretical arguments are identified. Notably, an unusual fibrillization pathway is also unraveled toward mixed-curvature polymorphs via the widespread recruitment and intertwining of protofilaments and protofibrils. The results present an original view of amyloid polymorphism and advance the fundamental understanding of the fibrillization mechanism from single protofilaments into mature amyloid fibrils.
中文翻译:

分层原丝缠绕规则混合曲率淀粉样蛋白多晶型物的形成
淀粉样蛋白多态性是几乎所有淀粉样蛋白种类的标志,但淀粉样蛋白多态性形成的机制及其复杂的结构仍然难以捉摸。通常,在以非零高斯曲率和平均曲率为特征的淀粉样蛋白多晶型物中发现两种主要的介观拓扑结构:分别是扭曲带和螺旋原纤维。在这里,在胰岛素淀粉样蛋白原纤维上展示了丰富的构型异质性,除了常见的多晶型物之外,还可以以形成混合曲率多晶型物的组合模式发生原丝堆积。通过 AFM 统计分析,确定了通过介观理论论证合理化的一系列异构架构。值得注意的是,通过原丝和原纤维的广泛募集和缠绕,一种不寻常的原纤维化途径也被揭示为混合曲率多晶型物。这些结果提出了淀粉样蛋白多态性的原始观点,并推进了对从单一原丝到成熟淀粉样蛋白原纤维的纤维化机制的基本理解。
更新日期:2024-06-20
中文翻译:

分层原丝缠绕规则混合曲率淀粉样蛋白多晶型物的形成
淀粉样蛋白多态性是几乎所有淀粉样蛋白种类的标志,但淀粉样蛋白多态性形成的机制及其复杂的结构仍然难以捉摸。通常,在以非零高斯曲率和平均曲率为特征的淀粉样蛋白多晶型物中发现两种主要的介观拓扑结构:分别是扭曲带和螺旋原纤维。在这里,在胰岛素淀粉样蛋白原纤维上展示了丰富的构型异质性,除了常见的多晶型物之外,还可以以形成混合曲率多晶型物的组合模式发生原丝堆积。通过 AFM 统计分析,确定了通过介观理论论证合理化的一系列异构架构。值得注意的是,通过原丝和原纤维的广泛募集和缠绕,一种不寻常的原纤维化途径也被揭示为混合曲率多晶型物。这些结果提出了淀粉样蛋白多态性的原始观点,并推进了对从单一原丝到成熟淀粉样蛋白原纤维的纤维化机制的基本理解。































 京公网安备 11010802027423号
京公网安备 11010802027423号