当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of ionic liquid (R4PSCN) for selective separation and recovery of copper from spent Cu[sbnd]Cr catalyst leach liquor
Hydrometallurgy ( IF 4.8 ) Pub Date : 2024-06-13 , DOI: 10.1016/j.hydromet.2024.106352 Saroj Sekhar Behera , Pankaj Kumar Parhi
Hydrometallurgy ( IF 4.8 ) Pub Date : 2024-06-13 , DOI: 10.1016/j.hydromet.2024.106352 Saroj Sekhar Behera , Pankaj Kumar Parhi
|
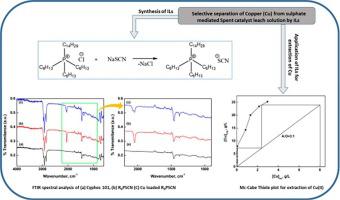
Separation and recovery of copper (Cu) from sulphate mediated CuCr spent catalyst leach solution through solvent extraction approach has been systematically investigated. A number of Ionic Liquid (IL) and other conventional extractants were used while investigating the selectivity and efficient extraction tendency of either IL or extractants towards Cu(II). In context of copper extraction efficiency, the adopted organic reagents followed the descending order: RPSCN > RPD > RPCy > Cyphos 101 > Aliquot 336 > D2EHPA > Cyanex 272. The extraction behavior of Cu(II) was established based on slope analysis method. From the results it was noticed that extraction occurs through a cation exchange mechanism with association of a mole of Cu(II) per mole of RPSCN. The plot of log D vs. log [RPSCN] yield a linear relationship with a slope close to 4 which is used to propose extraction reaction mechanism/ stoichiometry. The nature of complex between Cu(II) and IL was further examined using FTIR analysis of the loaded organic phase with the diluent. Mc-Cabe Thiele diagram was constructed to predict quantitative extraction of Cu(II) which revealed the need for two stages at aqueous to organic (A:O) phase volume ratio of =3:1. The stripping isotherm constructed at optimum NHOH concentration (0.4 M) suggests the need for two stages at O:A phase volume ratio of = 2:1 for complete stripping of copper with regeneration of RSCN for further use. Both isotherm conditions were validated by 6 cycles of counter current simulation (CCS) study for obtaining the desired amount of Cu(II) loaded RSCN (during extraction) and/or stripped copper solution (during stripping). Overall copper enrichment was ∼6 fold leading to produce a copper(II) solution of 48 g/L from 6 g/L Cu(II). The stripped solution was subjected to crystallization study to produce copper sulphate crystals of high purity and was confirmed by XRD analysis of crystal phases.
中文翻译:

离子液体(R4PSCN)对废Cu[sbnd]Cr催化剂浸出液中选择性分离回收铜的影响
通过溶剂萃取方法从硫酸盐介导的 CuCr 废催化剂浸出液中分离和回收铜 (Cu) 已进行了系统研究。在研究离子液体 (IL) 或萃取剂对 Cu(II) 的选择性和有效萃取趋势时,使用了多种离子液体 (IL) 和其他常规萃取剂。在铜萃取效率方面,所采用的有机试剂按降序排列:RPSCN > RPD > RPCy > Cyphos 101 > Aliquot 336 > D2EHPA > Cyanex 272。基于斜率分析方法建立了 Cu(II) 的萃取行为。从结果中可以看出,萃取是通过阳离子交换机制发生的,每摩尔 RPSCN 结合一摩尔 Cu(II)。 log D 与 log [RPSCN] 的关系图产生斜率接近 4 的线性关系,用于提出萃取反应机制/化学计量。使用稀释剂对负载的有机相进行 FTIR 分析,进一步检查 Cu(II) 和 IL 之间复合物的性质。构建 McCabe Thiele 图来预测 Cu(II) 的定量萃取,表明需要两个阶段,水相与有机相 (A:O) 体积比 = 3:1。在最佳 NHOH 浓度 (0.4 M) 下构建的剥离等温线表明,需要 O:A 相体积比 = 2:1 的两个阶段才能完全剥离铜,并再生 RSCN 以供进一步使用。两种等温条件均通过 6 个循环的逆流模拟 (CCS) 研究进行验证,以获得所需量的负载 Cu(II) 的 RSCN(萃取期间)和/或剥离的铜溶液(剥离期间)。总体铜富集度约为 6 倍,导致从 6 g/L Cu(II) 产生 48 g/L 的铜(II) 溶液。 对汽提后的溶液进行结晶研究,产生高纯度的硫酸铜晶体,并通过晶相的 XRD 分析证实。
更新日期:2024-06-13
中文翻译:

离子液体(R4PSCN)对废Cu[sbnd]Cr催化剂浸出液中选择性分离回收铜的影响
通过溶剂萃取方法从硫酸盐介导的 CuCr 废催化剂浸出液中分离和回收铜 (Cu) 已进行了系统研究。在研究离子液体 (IL) 或萃取剂对 Cu(II) 的选择性和有效萃取趋势时,使用了多种离子液体 (IL) 和其他常规萃取剂。在铜萃取效率方面,所采用的有机试剂按降序排列:RPSCN > RPD > RPCy > Cyphos 101 > Aliquot 336 > D2EHPA > Cyanex 272。基于斜率分析方法建立了 Cu(II) 的萃取行为。从结果中可以看出,萃取是通过阳离子交换机制发生的,每摩尔 RPSCN 结合一摩尔 Cu(II)。 log D 与 log [RPSCN] 的关系图产生斜率接近 4 的线性关系,用于提出萃取反应机制/化学计量。使用稀释剂对负载的有机相进行 FTIR 分析,进一步检查 Cu(II) 和 IL 之间复合物的性质。构建 McCabe Thiele 图来预测 Cu(II) 的定量萃取,表明需要两个阶段,水相与有机相 (A:O) 体积比 = 3:1。在最佳 NHOH 浓度 (0.4 M) 下构建的剥离等温线表明,需要 O:A 相体积比 = 2:1 的两个阶段才能完全剥离铜,并再生 RSCN 以供进一步使用。两种等温条件均通过 6 个循环的逆流模拟 (CCS) 研究进行验证,以获得所需量的负载 Cu(II) 的 RSCN(萃取期间)和/或剥离的铜溶液(剥离期间)。总体铜富集度约为 6 倍,导致从 6 g/L Cu(II) 产生 48 g/L 的铜(II) 溶液。 对汽提后的溶液进行结晶研究,产生高纯度的硫酸铜晶体,并通过晶相的 XRD 分析证实。

































 京公网安备 11010802027423号
京公网安备 11010802027423号