当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and anticancer assessment of structural analogues of (E)-1-((3,4,5-trimethoxybenzylidene)amino)-4-(3,4,5-trimethoxyphenyl)imidazo[1,2-a]quinoxaline-2-carbonitrile (6b), an imidazo[1,2-a]quinoxaline-based non-covalent EGFR inhibitor
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2024-06-20 , DOI: 10.1039/d4md00237g Manvendra Kumar 1 , Kiran T Patil 1 , Pritam Maity 1 , Joydeep Chatterjee 1 , Tashvinder Singh 2 , Gaurav Joshi 1, 3 , Sandeep Singh 2 , Raj Kumar 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2024-06-20 , DOI: 10.1039/d4md00237g Manvendra Kumar 1 , Kiran T Patil 1 , Pritam Maity 1 , Joydeep Chatterjee 1 , Tashvinder Singh 2 , Gaurav Joshi 1, 3 , Sandeep Singh 2 , Raj Kumar 1
Affiliation
|
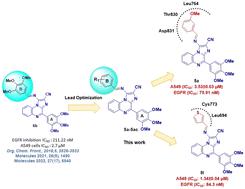
In our quest to find improved anticancer therapeutics, we expedite the lead optimization of (E)-1-((3,4,5-trimethoxybenzylidene)amino)-4-(3,4,5-trimethoxyphenyl)imidazo[1,2-a]quinoxaline-2-carbonitrile (6b), an EGFR inhibitor previously discovered in our laboratory through an in-house screening program. The lead optimization was rationally initiated considering the catalytic site of EGFR. We synthesized twenty-nine new analogues of 6b and assessed their anticancer activities. SAR studies highlighted the role of important groups in controlling anticancer activities. Among all, 5a and 5l were found to exhibit improved EGFR inhibition with anticancer asset potential. In silico studies corroborated with in vitro EGFR inhibitory results. The deeper analysis of 5a and 5l revealed that these synthetics could alter the MMP (ΔΨm) and significantly reduce the ROS levels in lung cancer cells. This is a vital prerequisite for better plausible EGFR inhibitors devoid of cardiotoxicity. qPCR analysis further revealed that the investigational compounds 5a and 5l were able to downregulate the expression of key oncogenes, viz., KRAS, MAP2K, and EGFR. The downregulation of these genes suggests that the investigational compounds could interact and inhibit key players in the signalling cascade along with the EGFR, which may lead to the inhibition of the growth and prognosis of cancer cells via a holistic approach.
中文翻译:

(E)-1-((3,4,5-三甲氧基亚苄基)氨基)-4-(3,4,5-三甲氧基苯基)咪唑并[1,2-a]喹喔啉结构类似物的设计、合成和抗癌评估-2-甲腈 (6b),一种基于咪唑并[1,2-a]喹喔啉的非共价 EGFR 抑制剂
在寻找改进的抗癌疗法的过程中,我们加快了 ( E )-1-((3,4,5-三甲氧基亚苄基)氨基)-4-(3,4,5-三甲氧基苯基)咪唑[1,2]的先导化合物优化- a ]喹喔啉-2-甲腈 ( 6b ),这是我们实验室之前通过内部筛选计划发现的一种 EGFR 抑制剂。考虑EGFR的催化位点,合理启动先导化合物优化。我们合成了 29 种新的6b类似物并评估了它们的抗癌活性。 SAR 研究强调了重要群体在控制抗癌活动中的作用。其中, 5a和5l被发现表现出改善的 EGFR 抑制作用,并具有抗癌潜力。计算机模拟研究证实了体外EGFR 抑制结果。对5a和5l的更深入分析表明,这些合成物可以改变 MMP (Δ Ψ m ) 并显着降低肺癌细胞中的 ROS 水平。这是开发更合理且无心脏毒性的 EGFR 抑制剂的重要先决条件。 qPCR 分析进一步表明,研究化合物5a和5l能够下调关键癌基因的表达,即。 、KRAS、MAP2K 和 EGFR。这些基因的下调表明,研究化合物可以与 EGFR 一起相互作用并抑制信号级联中的关键参与者,这可能通过整体方法抑制癌细胞的生长和预后。
更新日期:2024-06-20
中文翻译:

(E)-1-((3,4,5-三甲氧基亚苄基)氨基)-4-(3,4,5-三甲氧基苯基)咪唑并[1,2-a]喹喔啉结构类似物的设计、合成和抗癌评估-2-甲腈 (6b),一种基于咪唑并[1,2-a]喹喔啉的非共价 EGFR 抑制剂
在寻找改进的抗癌疗法的过程中,我们加快了 ( E )-1-((3,4,5-三甲氧基亚苄基)氨基)-4-(3,4,5-三甲氧基苯基)咪唑[1,2]的先导化合物优化- a ]喹喔啉-2-甲腈 ( 6b ),这是我们实验室之前通过内部筛选计划发现的一种 EGFR 抑制剂。考虑EGFR的催化位点,合理启动先导化合物优化。我们合成了 29 种新的6b类似物并评估了它们的抗癌活性。 SAR 研究强调了重要群体在控制抗癌活动中的作用。其中, 5a和5l被发现表现出改善的 EGFR 抑制作用,并具有抗癌潜力。计算机模拟研究证实了体外EGFR 抑制结果。对5a和5l的更深入分析表明,这些合成物可以改变 MMP (Δ Ψ m ) 并显着降低肺癌细胞中的 ROS 水平。这是开发更合理且无心脏毒性的 EGFR 抑制剂的重要先决条件。 qPCR 分析进一步表明,研究化合物5a和5l能够下调关键癌基因的表达,即。 、KRAS、MAP2K 和 EGFR。这些基因的下调表明,研究化合物可以与 EGFR 一起相互作用并抑制信号级联中的关键参与者,这可能通过整体方法抑制癌细胞的生长和预后。































 京公网安备 11010802027423号
京公网安备 11010802027423号