当前位置:
X-MOL 学术
›
Mol. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
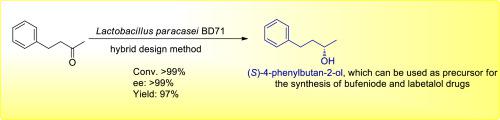
Efficient bioreduction of 4-phenyl-2-butanone to drug precursor (S)-4-phenyl-2-butanol by a whole-cell biocatalyst using a novel hybrid design technique
Molecular Catalysis ( IF 3.9 ) Pub Date : 2024-06-08 , DOI: 10.1016/j.mcat.2024.114289 Beyzanur Bayhan , Akın Özdemir , Enes Dertli , Engin Şahin
Molecular Catalysis ( IF 3.9 ) Pub Date : 2024-06-08 , DOI: 10.1016/j.mcat.2024.114289 Beyzanur Bayhan , Akın Özdemir , Enes Dertli , Engin Şahin
|
Asymmetric synthesis is a critical tactic in pharmaceutical industries for creating chiral medications as it allows an enantiomer to be obtained in synthetic chemistry. The asymmetric bioreduction processes by biocatalysts have shown significant potential in producing chiral alcohols. The amount of substrate and the production method of the biocatalytic synthesis of ()-4-phenyl-2-butanol () are not still desired levels. Furthermore, the biocatalytic asymmetric reduction of 4-phenyl-2-butanone () to ()- or ()-4-phenyl-2-butanol did not use any mathematical modeling techniques. In this study, the asymmetric bioreduction of was carried out in this work employing BD71 biocatalyst and a novel hybrid design-based optimization approach. By using the hybrid design technique, the optimal circumstances were discovered to be pH = 7, temperature = 29 °C, incubation period = 66 h, and agitation speed = 189 rpm. Also, the enantiomeric excess (ee) and conversion could be 99.15 % and 98.19 %, respectively. Next, was acquired to be ee: 99 %, conversion: >99 %, and yield: 97 % from the optimum bioreduction conditions. Furthermore, 14.08 g of 1 under optimal conditions was entirely transformed into (13.84 g, 97 % isolated yield). This study is the first research attempt to use a biocatalyst and an innovative and new hybrid design-based optimization approach to fabricate enantiopure at a high gram scale. This work has successfully demonstrated that the new hybrid design-based optimization technique is applicable to biocatalytic asymmetric reduction processes.
中文翻译:

使用新型混合设计技术,通过全细胞生物催化剂将 4-苯基-2-丁酮有效生物还原为药物前体 (S)-4-苯基-2-丁醇
不对称合成是制药工业中制造手性药物的关键策略,因为它允许在合成化学中获得对映体。生物催化剂的不对称生物还原过程在生产手性醇方面显示出巨大的潜力。生物催化合成()-4-苯基-2-丁醇()的底物量和生产方法仍达不到理想的水平。此外,4-苯基-2-丁酮()的生物催化不对称还原为()-或()-4-苯基-2-丁醇没有使用任何数学建模技术。在这项研究中,采用 BD71 生物催化剂和一种新型的基于混合设计的优化方法进行了不对称生物还原。通过使用混合设计技术,发现最佳环境为 pH = 7、温度 = 29 °C、孵育时间 = 66 小时、搅拌速度 = 189 rpm。此外,对映体过量 (ee) 和转化率可分别为 99.15% 和 98.19%。接下来,从最佳生物还原条件获得ee:99%,转化率:>99%,产率:97%。此外,在最佳条件下,14.08 g 1 被完全转化(13.84 g,分离产率 97%)。这项研究是首次研究尝试使用生物催化剂和基于创新的混合设计的优化方法来制造高克规模的对映体纯。这项工作成功证明了基于混合设计的新优化技术适用于生物催化不对称还原过程。
更新日期:2024-06-08
中文翻译:

使用新型混合设计技术,通过全细胞生物催化剂将 4-苯基-2-丁酮有效生物还原为药物前体 (S)-4-苯基-2-丁醇
不对称合成是制药工业中制造手性药物的关键策略,因为它允许在合成化学中获得对映体。生物催化剂的不对称生物还原过程在生产手性醇方面显示出巨大的潜力。生物催化合成()-4-苯基-2-丁醇()的底物量和生产方法仍达不到理想的水平。此外,4-苯基-2-丁酮()的生物催化不对称还原为()-或()-4-苯基-2-丁醇没有使用任何数学建模技术。在这项研究中,采用 BD71 生物催化剂和一种新型的基于混合设计的优化方法进行了不对称生物还原。通过使用混合设计技术,发现最佳环境为 pH = 7、温度 = 29 °C、孵育时间 = 66 小时、搅拌速度 = 189 rpm。此外,对映体过量 (ee) 和转化率可分别为 99.15% 和 98.19%。接下来,从最佳生物还原条件获得ee:99%,转化率:>99%,产率:97%。此外,在最佳条件下,14.08 g 1 被完全转化(13.84 g,分离产率 97%)。这项研究是首次研究尝试使用生物催化剂和基于创新的混合设计的优化方法来制造高克规模的对映体纯。这项工作成功证明了基于混合设计的新优化技术适用于生物催化不对称还原过程。







































 京公网安备 11010802027423号
京公网安备 11010802027423号