当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparison of selectivity and sensitivity of various ferric selective electrodes prepared using N-((bis(dimethyl amino)methylene)carbamothioyl)benzamide
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-06-18 , DOI: 10.1039/d4cp01473a Mehrdad Ghaemi 1 , Leila Hajiaghababaei 1 , Jamshid Najafpour 1 , Ashraf Sadat Shahvelayati 1 , Ramin M A Tehrani 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-06-18 , DOI: 10.1039/d4cp01473a Mehrdad Ghaemi 1 , Leila Hajiaghababaei 1 , Jamshid Najafpour 1 , Ashraf Sadat Shahvelayati 1 , Ramin M A Tehrani 1
Affiliation
|
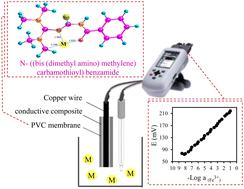
N-((Bis(dimethyl amino)methylene)carbamothioyl)benzamide (NBMCB) was synthesized, characterized, and used as an ionophore for producing three novel ion-selective potentiometric sensors for Fe(III) determination. Firstly, using the molecular mechanic-based MMFF94 method, the most stable NBMCB's conformer and its isosteric complexes with various cations were determined. According to the Gibbs free energy results of the reaction, the thermodynamic complexation reactivity of Fe(III) and the ligand was acceptable. These results were obtained using the B3LYP approach and the 6-31G(d,p) basis set that was substituted for heavy metals by the LanL2DZ basis set. We used UV-visible spectrophotometry to confirm the tendency of NBMCB to react with Fe(III). Generally, three diverse liquid membrane ferric selective electrodes were obtained by the use of the specified ligand: classic with a liquid internal electrolyte-ferric selective electrode (LIE-FSE), solid state-FSE (SS-FSE), and coated wire-FSE (CW-FSE). The reactions exhibited Nernstian behavior across all electrodes. The limit of detection was enhanced for the SS-FSE (3 × 10−9 M) and the CW-FSE (3 × 10−7 M) in comparison with that of the LIE-FSE (7 × 10−7 M). The lifetime of the LIE-FSE was 8 weeks, while it was 10 weeks for the SS-FSE and the CW-FSE. Elimination of the internal solution reduced the limit of detection and prolonged the lifespan of the sensors. Also, the three electrodes all had a short response time of around 5–7 s. The sensors were utilized as indicator electrodes during the potentiometric titration of Fe(III) using ethylenediaminetetraacetic acid.
中文翻译:

N-((双(二甲氨基)亚甲基)硫氨甲酰基)苯甲酰胺制备的各种铁选择性电极的选择性和灵敏度比较
合成、表征N -((双(二甲基氨基)亚甲基)硫代氨基甲酰基)苯甲酰胺 (NBMCB),并将其用作离子载体来生产三种用于 Fe( III ) 测定的新型离子选择性电位传感器。首先,利用基于分子力学的MMFF94方法,确定了最稳定的NBMCB构象异构体及其与各种阳离子的等排配合物。根据反应的吉布斯自由能结果,Fe( III )与配体的热力学络合反应活性良好。这些结果是使用 B3LYP 方法和 6-31G(d,p) 基组获得的,该基组由 LanL2DZ 基组替代重金属。我们使用紫外可见分光光度法证实了NBMCB与Fe( III )反应的趋势。一般来说,通过使用指定的配体获得了三种不同的液膜铁选择性电极:经典的液体内部电解质铁选择性电极(LIE-FSE)、固态FSE(SS-FSE)和涂层线FSE (CW-FSE)。所有电极上的反应都表现出能斯特行为。与LIE-FSE (7 × 10 -7 M) 相比,SS-FSE (3 × 10 -9 M) 和CW-FSE (3 × 10 -7 M) 的检测限有所提高。 LIE-FSE 的寿命为 8 周,而 SS-FSE 和 CW-FSE 的寿命为 10 周。消除内部解决方案降低了检测限并延长了传感器的使用寿命。此外,三个电极的响应时间都很短,约为 5-7 秒。 在使用乙二胺四乙酸进行 Fe( III ) 电位滴定过程中,传感器用作指示电极。
更新日期:2024-06-18
中文翻译:

N-((双(二甲氨基)亚甲基)硫氨甲酰基)苯甲酰胺制备的各种铁选择性电极的选择性和灵敏度比较
合成、表征N -((双(二甲基氨基)亚甲基)硫代氨基甲酰基)苯甲酰胺 (NBMCB),并将其用作离子载体来生产三种用于 Fe( III ) 测定的新型离子选择性电位传感器。首先,利用基于分子力学的MMFF94方法,确定了最稳定的NBMCB构象异构体及其与各种阳离子的等排配合物。根据反应的吉布斯自由能结果,Fe( III )与配体的热力学络合反应活性良好。这些结果是使用 B3LYP 方法和 6-31G(d,p) 基组获得的,该基组由 LanL2DZ 基组替代重金属。我们使用紫外可见分光光度法证实了NBMCB与Fe( III )反应的趋势。一般来说,通过使用指定的配体获得了三种不同的液膜铁选择性电极:经典的液体内部电解质铁选择性电极(LIE-FSE)、固态FSE(SS-FSE)和涂层线FSE (CW-FSE)。所有电极上的反应都表现出能斯特行为。与LIE-FSE (7 × 10 -7 M) 相比,SS-FSE (3 × 10 -9 M) 和CW-FSE (3 × 10 -7 M) 的检测限有所提高。 LIE-FSE 的寿命为 8 周,而 SS-FSE 和 CW-FSE 的寿命为 10 周。消除内部解决方案降低了检测限并延长了传感器的使用寿命。此外,三个电极的响应时间都很短,约为 5-7 秒。 在使用乙二胺四乙酸进行 Fe( III ) 电位滴定过程中,传感器用作指示电极。































 京公网安备 11010802027423号
京公网安备 11010802027423号