当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric catalytic [1,3]- or [3,3]-sigmatropic rearrangement of 3-allyloxy-4H-chromenones and their analogues
Chemical Science ( IF 7.6 ) Pub Date : 2024-06-19 , DOI: 10.1039/d4sc02201g Yi Li 1 , Lichao Ning 1 , Qi Tang 1 , Kexin Lan 1 , Bingqian Yang 1 , Qianchi Lin 1 , Xiaoming Feng 1 , Xiaohua Liu 1
Chemical Science ( IF 7.6 ) Pub Date : 2024-06-19 , DOI: 10.1039/d4sc02201g Yi Li 1 , Lichao Ning 1 , Qi Tang 1 , Kexin Lan 1 , Bingqian Yang 1 , Qianchi Lin 1 , Xiaoming Feng 1 , Xiaohua Liu 1
Affiliation
|
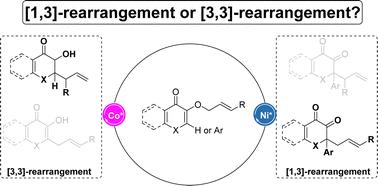
A highly efficient asymmetric [1,3]- and [3,3]-O-to-C sigmatropic rearrangement of 3-allyloxy-4H-chromenones and their analogues was developed. Chiral N,N′-dioxide complexes of 3d late transition metal complexes enabled two mechanistically different processes, giving a series of optically active 2,2-disubstituted chromane-3,4-diones and 2-allyl-3-hydroxy-4H-chromen-4-ones as well as their related compounds in excellent yield and enantioselectivity. Systemic mechanistic studies and DFT calculation revealed the nature of the vinyl ether unit of the substrate, which biased regioselectivity via a stepwise tight ion pair pathway and a concerted pericyclic pathway, respectively. The enantioselectivity of the two processes is also disclosed.
中文翻译:

3-烯丙氧基-4H-色酮及其类似物的不对称催化[1,3]-或[3,3]-σ重排
开发了 3-烯丙氧基-4 H-色酮及其类似物的高效不对称 [1,3]- 和 [3,3]-O-to-C 重排。 3d后过渡金属配合物的手性N , N'-二氧化物配合物实现了两种机械上不同的过程,给出了一系列光学活性的2,2-二取代色满-3,4-二酮和2-烯丙基-3-羟基-4 H- chromen-4-ones 及其相关化合物具有优异的产率和对映选择性。系统机理研究和DFT计算揭示了底物乙烯基醚单元的性质,其分别通过逐步紧密离子对路径和协同周环路径偏向区域选择性。还公开了这两种方法的对映选择性。
更新日期:2024-06-19
中文翻译:

3-烯丙氧基-4H-色酮及其类似物的不对称催化[1,3]-或[3,3]-σ重排
开发了 3-烯丙氧基-4 H-色酮及其类似物的高效不对称 [1,3]- 和 [3,3]-O-to-C 重排。 3d后过渡金属配合物的手性N , N'-二氧化物配合物实现了两种机械上不同的过程,给出了一系列光学活性的2,2-二取代色满-3,4-二酮和2-烯丙基-3-羟基-4 H- chromen-4-ones 及其相关化合物具有优异的产率和对映选择性。系统机理研究和DFT计算揭示了底物乙烯基醚单元的性质,其分别通过逐步紧密离子对路径和协同周环路径偏向区域选择性。还公开了这两种方法的对映选择性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号