当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crucial Effect of Subsurface Hydrogen on Low-Barrier Hydrogenation and Keto–Enol Tautomerization of Carbonyl Compounds
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-06-19 , DOI: 10.1021/acscatal.4c01441
Philipp A. Haugg 1 , Jan Smyczek 1 , Patrick Hubert 1 , Carsten Schröder 1 , Swetlana Schauermann 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-06-19 , DOI: 10.1021/acscatal.4c01441
Philipp A. Haugg 1 , Jan Smyczek 1 , Patrick Hubert 1 , Carsten Schröder 1 , Swetlana Schauermann 1
Affiliation
|
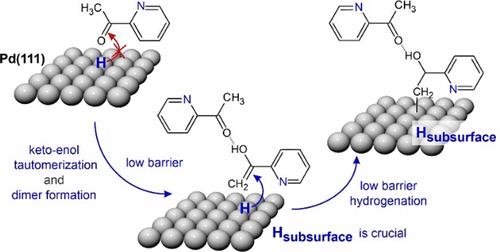
Hydrogenation of a normally highly stable carbonyl group is an important step in many technological applications, including emerging molecular systems for reversible hydrogen storage. In this report, we present a mechanistic study of low-temperature hydrogenation of carbonyl compounds over Pd, proceeding via keto–enol tautomerization in the first step. The specific focus of this study is on the role of subsurface hydrogen absorbed in the nearest region below the Pd surface. Employing a combination of real space microscopic and operando spectroscopic surface sensitive techniques, as well as molecular beams, we show that subsurface H plays a crucial role in both keto–enol tautomerization and hydrogenation of the carbonyl compound acetylpyridine on the Pd(111) model catalyst. We demonstrate that a growing amount of subsurface H results in an enhanced abundance of enol species followed by hydrogenation already at cryogenic temperatures. In contrast, if only H adsorbed on the surface is present, no hydrogenation occurs, and substantially smaller amounts of enol species are formed. The population of subsurface H is also accompanied by a change in the mechanism of enol stabilization via hydrogen bonding: while in the presence of subsurface H specific enol-containing dimers are predominately formed, which strongly interact via the enol-acetyl or enol–enol groups, in the absence of subsurface H, a weaker interaction between the adsorbates occurs, which is realized mainly via the enol group of one molecule with a H atom belonging to the pyridine ring of the neighboring adsorbate. The observed strongly correlated behavior between the growing concentration of subsurface H, enhanced abundance of the enol form of acetylpyridine, and the onset of hydrogenation prove the crucial role of subsurface H species in the low-barrier hydrogenation pathway of carbonyl compounds. The obtained atomistic-level insights offer a prospect of controllable low-temperature hydrogenation of carbonyl compounds by tuning the abundance of subsurface H, which has not been available so far.
中文翻译:

地下氢对羰基化合物低势垒氢化和酮-烯醇互变异构的关键影响
通常高度稳定的羰基的氢化是许多技术应用中的重要一步,包括用于可逆储氢的新兴分子系统。在本报告中,我们提出了羰基化合物在钯上低温氢化的机理研究,第一步是通过酮-烯醇互变异构化进行。这项研究的具体重点是 Pd 表面以下最近区域吸收的地下氢的作用。结合实空间显微技术和原操作光谱表面敏感技术以及分子束,我们发现次表面H在Pd(111)模型催化剂上羰基化合物乙酰吡啶的酮-烯醇互变异构和氢化中起着至关重要的作用。我们证明,地下氢含量的增加会导致烯醇种类的丰度增加,然后在低温下氢化。相反,如果仅存在吸附在表面上的H,则不会发生氢化,并且形成显着更少量的烯醇物质。地下 H 的数量还伴随着通过氢键作用的烯醇稳定机制的变化:而在地下 H 存在的情况下,主要形成特定的含烯醇二聚体,它们通过烯醇-乙酰基或烯醇-烯醇基团强烈相互作用,在没有次表面 H 的情况下,吸附物之间会发生较弱的相互作用,这主要是通过一个分子的烯醇基团与属于相邻吸附物的吡啶环的 H 原子实现的。 观察到的地下氢浓度的增加、乙酰吡啶烯醇形式丰度的增加以及氢化的开始之间的强相关行为证明了地下氢物种在羰基化合物的低势垒氢化途径中的关键作用。所获得的原子水平的见解为通过调节地下H的丰度来实现羰基化合物的可控低温氢化提供了前景,但迄今为止尚未实现。
更新日期:2024-06-19
中文翻译:

地下氢对羰基化合物低势垒氢化和酮-烯醇互变异构的关键影响
通常高度稳定的羰基的氢化是许多技术应用中的重要一步,包括用于可逆储氢的新兴分子系统。在本报告中,我们提出了羰基化合物在钯上低温氢化的机理研究,第一步是通过酮-烯醇互变异构化进行。这项研究的具体重点是 Pd 表面以下最近区域吸收的地下氢的作用。结合实空间显微技术和原操作光谱表面敏感技术以及分子束,我们发现次表面H在Pd(111)模型催化剂上羰基化合物乙酰吡啶的酮-烯醇互变异构和氢化中起着至关重要的作用。我们证明,地下氢含量的增加会导致烯醇种类的丰度增加,然后在低温下氢化。相反,如果仅存在吸附在表面上的H,则不会发生氢化,并且形成显着更少量的烯醇物质。地下 H 的数量还伴随着通过氢键作用的烯醇稳定机制的变化:而在地下 H 存在的情况下,主要形成特定的含烯醇二聚体,它们通过烯醇-乙酰基或烯醇-烯醇基团强烈相互作用,在没有次表面 H 的情况下,吸附物之间会发生较弱的相互作用,这主要是通过一个分子的烯醇基团与属于相邻吸附物的吡啶环的 H 原子实现的。 观察到的地下氢浓度的增加、乙酰吡啶烯醇形式丰度的增加以及氢化的开始之间的强相关行为证明了地下氢物种在羰基化合物的低势垒氢化途径中的关键作用。所获得的原子水平的见解为通过调节地下H的丰度来实现羰基化合物的可控低温氢化提供了前景,但迄今为止尚未实现。




































 京公网安备 11010802027423号
京公网安备 11010802027423号