Cell Metabolism ( IF 27.7 ) Pub Date : 2024-06-18 , DOI: 10.1016/j.cmet.2024.05.013 Yu Jiang 1 , Yawen Wang 1 , Guofeng Chen 1 , Fei Sun 1 , Qijing Wu 1 , Qiong Huang 1 , Dongqiang Zeng 1 , Wenjun Qiu 1 , Jiao Wang 1 , Zhiqi Yao 1 , Bishan Liang 1 , Shaowei Li 1 , Jianhua Wu 1 , Na Huang 1 , Yuanyuan Wang 1 , Jingsong Chen 2 , Xiaohui Zhai 3 , Li Huang 4 , Beibei Xu 1 , Masami Yamamoto 5 , Tetsuya Tsukamoto 6 , Sachiyo Nomura 7 , Wangjun Liao 8 , Min Shi 1
|
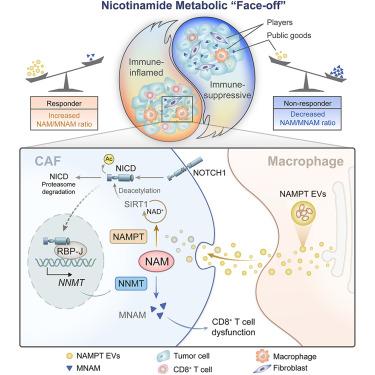
Immune checkpoint blockade has led to breakthroughs in the treatment of advanced gastric cancer. However, the prominent heterogeneity in gastric cancer, notably the heterogeneity of the tumor microenvironment, highlights the idea that the antitumor response is a reflection of multifactorial interactions. Through transcriptomic analysis and dynamic plasma sample analysis, we identified a metabolic “face-off” mechanism within the tumor microenvironment, as shown by the dual prognostic significance of nicotinamide metabolism. Specifically, macrophages and fibroblasts expressing the rate-limiting enzymes nicotinamide phosphoribosyltransferase and nicotinamide N-methyltransferase, respectively, regulate the nicotinamide/1-methylnicotinamide ratio and CD8+ T cell function. Mechanistically, nicotinamide N-methyltransferase is transcriptionally activated by the NOTCH pathway transcription factor RBP-J and is further inhibited by macrophage-derived extracellular vesicles containing nicotinamide phosphoribosyltransferase via the SIRT1/NICD axis. Manipulating nicotinamide metabolism through autologous injection of extracellular vesicles restored CD8+ T cell cytotoxicity and the anti-PD-1 response in gastric cancer.
中文翻译:

巨噬细胞和成纤维细胞之间的烟酰胺代谢对抗操纵胃癌的微环境
免疫检查点阻断导致晚期胃癌治疗取得突破。然而,胃癌显着的异质性,特别是肿瘤微环境的异质性,凸显了抗肿瘤反应是多因素相互作用反映的观点。通过转录组分析和动态血浆样本分析,我们确定了肿瘤微环境内的代谢“对峙”机制,烟酰胺代谢的双重预后意义就表明了这一点。具体而言,巨噬细胞和成纤维细胞分别表达限速酶烟酰胺磷酸核糖基转移酶和烟酰胺N-甲基转移酶,调节烟酰胺/1-甲基烟酰胺比率和CD8 + T细胞功能。从机制上讲,烟酰胺 N-甲基转移酶由 NOTCH 通路转录因子 RBP-J 转录激活,并通过 SIRT1/NICD 轴进一步被巨噬细胞衍生的含有烟酰胺磷酸核糖基转移酶的细胞外囊泡抑制。通过自体注射细胞外囊泡控制烟酰胺代谢可恢复胃癌中 CD8 + T 细胞的细胞毒性和抗 PD-1 反应。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号