当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Directing group controlled regioselective C–H borylation of 2-arylphenolic compounds at room temperature
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-18 , DOI: 10.1039/d4qo00805g Jiao Kang 1 , Jing Liu 1 , Zhilong Chen 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-18 , DOI: 10.1039/d4qo00805g Jiao Kang 1 , Jing Liu 1 , Zhilong Chen 1
Affiliation
|
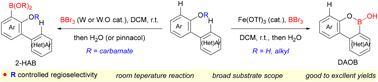
Regioselective borylation of (hetero)arenes under mild conditions is an appealing strategy for constructing boron-containing compounds, particularly considering their broad applications in organic synthesis, medicinal chemistry and material science. In this manuscript, we developed a Fe(OTf)3-promoted, DG (directing group)-controlled, regioselective electrophilic C–H borylation of 2-arylphenolic compounds with BBr3 at room temperature. C2′-borylation was dramatically accelerated by an LA-catalyst, affording DAOB (diaryloxaborin) with a broad substrate scope in moderate-to-excellent yields. The selective ortho-borylation was successfully achieved by introducing phenolic carbamate as a directing group to overcome the competing Fries rearrangement, giving desired 2-HAB ((2-hydroxyaryl)boronic acid) in good yields for most substrates.
中文翻译:

室温下 2-芳基酚类化合物的定向基团控制区域选择性 C-H 硼基化
在温和条件下(杂)芳烃的区域选择性硼化是构建含硼化合物的一种有吸引力的策略,特别是考虑到它们在有机合成、药物化学和材料科学中的广泛应用。在这篇手稿中,我们开发了一种 Fe(OTf) 3 促进、DG(导向基团)控制、区域选择性亲电 C-H 2-芳基酚化合物与 BBr 3 的硼基化反应,反应温度为室内温度。 LA 催化剂极大地加速了 C2'-硼基化,提供了具有广泛底物范围的 DAOB(二芳基恶硼啉),产率中等至优异。通过引入酚氨基甲酸酯作为导向基团以克服竞争性 Fries 重排,成功实现了选择性邻硼酸化,从而对大多数底物以良好的产率产生了所需的 2-HAB((2-羟基芳基)硼酸)。
更新日期:2024-06-18
中文翻译:

室温下 2-芳基酚类化合物的定向基团控制区域选择性 C-H 硼基化
在温和条件下(杂)芳烃的区域选择性硼化是构建含硼化合物的一种有吸引力的策略,特别是考虑到它们在有机合成、药物化学和材料科学中的广泛应用。在这篇手稿中,我们开发了一种 Fe(OTf) 3 促进、DG(导向基团)控制、区域选择性亲电 C-H 2-芳基酚化合物与 BBr 3 的硼基化反应,反应温度为室内温度。 LA 催化剂极大地加速了 C2'-硼基化,提供了具有广泛底物范围的 DAOB(二芳基恶硼啉),产率中等至优异。通过引入酚氨基甲酸酯作为导向基团以克服竞争性 Fries 重排,成功实现了选择性邻硼酸化,从而对大多数底物以良好的产率产生了所需的 2-HAB((2-羟基芳基)硼酸)。






































 京公网安备 11010802027423号
京公网安备 11010802027423号