当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One-pot multistep synthesis of 1-fluoroalkylisoquinolines and fused fluoroalkylpyridines from N-fluoroalkyl-1,2,3-triazoles
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-18 , DOI: 10.1039/d4qo00713a Anna Kubíčková 1, 2 , Svatava Voltrová 1 , Adam Kleman 1 , Blanka Klepetářová 1 , Petr Beier 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-18 , DOI: 10.1039/d4qo00713a Anna Kubíčková 1, 2 , Svatava Voltrová 1 , Adam Kleman 1 , Blanka Klepetářová 1 , Petr Beier 1
Affiliation
|
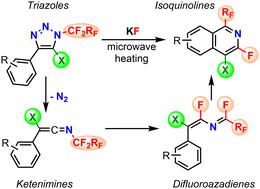
An efficient one-pot microwave-assisted potassium fluoride-mediated synthesis of 1-fluoroalkyl-3-fluoroisoquinolines and fused fluoroalkylpyridines from N-fluoroalkylated 1,2,3-triazoles was developed. The reaction has a wide scope and allows the preparation of structurally diverse 3-fluoroisoquinolines with a fluoroalkyl group in position 1, a substituent in position 4 and a substituent on the fused benzene (or heteroaromatic) ring. N-Fluoroalkylated ketenimines, which undergo stereoselective formal 1,3-fluorine shift to difluoroazadienes, were identified as intermediates in the reaction sequence. The presence of fluorine in position 3 and a halogen in position 4 of the resulting isoquinolines allowed for further modification by nucleophilic aromatic substitution and cross-coupling reactions, respectively. The developed methodologies were utilized for the synthesis of derivatives of drug candidates.
中文翻译:

N-氟烷基-1,2,3-三唑一锅多步合成1-氟烷基异喹啉和稠合氟烷基吡啶
开发了一种高效的一锅微波辅助氟化钾介导的从 N-氟烷基化 1,2,3-三唑合成 1-氟烷基-3-氟异喹啉和稠合氟烷基吡啶的方法。该反应范围广泛,可以制备结构多样的3-氟异喹啉,其中1位有氟烷基,4位有取代基,稠合苯(或杂芳族)环上有取代基。 N-氟烷基化烯酮亚胺,其经历立体选择性形式1,3-氟转变为二氟氮杂二烯,被鉴定为反应序列中的中间体。所得异喹啉的 3 位存在氟,4 位存在卤素,可以分别通过亲核芳族取代和交叉偶联反应进行进一步修饰。开发的方法用于候选药物衍生物的合成。
更新日期:2024-06-18
中文翻译:

N-氟烷基-1,2,3-三唑一锅多步合成1-氟烷基异喹啉和稠合氟烷基吡啶
开发了一种高效的一锅微波辅助氟化钾介导的从 N-氟烷基化 1,2,3-三唑合成 1-氟烷基-3-氟异喹啉和稠合氟烷基吡啶的方法。该反应范围广泛,可以制备结构多样的3-氟异喹啉,其中1位有氟烷基,4位有取代基,稠合苯(或杂芳族)环上有取代基。 N-氟烷基化烯酮亚胺,其经历立体选择性形式1,3-氟转变为二氟氮杂二烯,被鉴定为反应序列中的中间体。所得异喹啉的 3 位存在氟,4 位存在卤素,可以分别通过亲核芳族取代和交叉偶联反应进行进一步修饰。开发的方法用于候选药物衍生物的合成。






































 京公网安备 11010802027423号
京公网安备 11010802027423号