当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photocatalyzed sulfoximination/amidation of (Het)arylethenes tethered N-tosyl amide: a versatile entry to sulfoximidoyl β- and γ-lactams
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-18 , DOI: 10.1039/d4qo00631c Chengtan Li 1 , Yuming Yang 1 , Xiaolan Zheng 1 , Cairong Zhang 1 , Hui Cai 1 , Weilong Lin 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-18 , DOI: 10.1039/d4qo00631c Chengtan Li 1 , Yuming Yang 1 , Xiaolan Zheng 1 , Cairong Zhang 1 , Hui Cai 1 , Weilong Lin 1
Affiliation
|
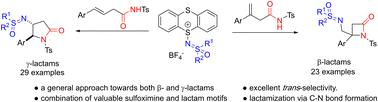
Lactam and sulfoximine motifs are common in natural and biologically active molecules, and alkenes are important building blocks in synthetic chemistry. Through photoredox-mediated formation of cation intermediates, we have developed a highly versatile method for preparing various sulfoximidoyl lactams via C–N bond formation between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and amide motifs. Mild reaction conditions, broad functional group tolerance and excellent trans-selectivity of this method would make it a general and efficient approach towards novel compounds containing valuable sulfoximine and lactam motifs for possible therapeutic use.
C and amide motifs. Mild reaction conditions, broad functional group tolerance and excellent trans-selectivity of this method would make it a general and efficient approach towards novel compounds containing valuable sulfoximine and lactam motifs for possible therapeutic use.
中文翻译:

(Het)arylethenes 系链 N-甲苯磺酰酰胺的光催化磺酰基化/酰胺化:亚磺酰亚胺酰基 β- 和 γ-内酰胺的多功能入口
内酰胺和亚砜亚胺基序在天然和生物活性分子中很常见,而烯烃是合成化学中的重要组成部分。通过光氧化还原介导的阳离子中间体的形成,我们开发了一种高度通用的方法,通过 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C 和酰胺基序之间形成 C-N 键来制备各种亚磺酰亚胺酰内酰胺。该方法温和的反应条件、广泛的官能团耐受性和优异的反式选择性将使其成为一种通用且有效的方法,用于制备含有有价值的亚砜亚胺和内酰胺基序的新型化合物,用于可能的治疗用途。
C 和酰胺基序之间形成 C-N 键来制备各种亚磺酰亚胺酰内酰胺。该方法温和的反应条件、广泛的官能团耐受性和优异的反式选择性将使其成为一种通用且有效的方法,用于制备含有有价值的亚砜亚胺和内酰胺基序的新型化合物,用于可能的治疗用途。
更新日期:2024-06-18
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and amide motifs. Mild reaction conditions, broad functional group tolerance and excellent trans-selectivity of this method would make it a general and efficient approach towards novel compounds containing valuable sulfoximine and lactam motifs for possible therapeutic use.
C and amide motifs. Mild reaction conditions, broad functional group tolerance and excellent trans-selectivity of this method would make it a general and efficient approach towards novel compounds containing valuable sulfoximine and lactam motifs for possible therapeutic use.
中文翻译:

(Het)arylethenes 系链 N-甲苯磺酰酰胺的光催化磺酰基化/酰胺化:亚磺酰亚胺酰基 β- 和 γ-内酰胺的多功能入口
内酰胺和亚砜亚胺基序在天然和生物活性分子中很常见,而烯烃是合成化学中的重要组成部分。通过光氧化还原介导的阳离子中间体的形成,我们开发了一种高度通用的方法,通过 C






































 京公网安备 11010802027423号
京公网安备 11010802027423号