当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photo-induced synthesis of fluoroalkylated quinolinones via an iron-catalyzed LMCT decarboxylation process
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-18 , DOI: 10.1039/d4qo00707g Zhuoheng Song 1 , Lin Guo 1 , Chao Yang 1 , Wujiong Xia 1, 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-18 , DOI: 10.1039/d4qo00707g Zhuoheng Song 1 , Lin Guo 1 , Chao Yang 1 , Wujiong Xia 1, 2
Affiliation
|
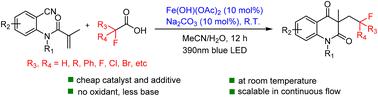
Herein, we report a practical synthetic method for the efficient preparation of fluoroalkylated quinoline-2,4-diones using N-(2-cyanophenyl)-N-methylacrylamides as substrates, fluoroalkyl carboxylic acids as reactants, and Fe(OH)(OAc)2 as a catalyst. This reaction started with a photo-driven ligand-to-metal charge transfer (LMCT) process to decarboxylate the reactant and produce fluoroalkyl radicals, which then underwent radical cascade cyclization with the substrate. The target products were obtained in 55–91% yields. A gram scale experiment was also completed using a continuous-flow photocatalytic device.
中文翻译:

通过铁催化 LMCT 脱羧过程光诱导合成氟烷基喹啉酮
在此,我们报道了一种以 N-(2-氰基苯基)-N-甲基丙烯酰胺为底物、氟烷基羧酸为反应物和 Fe(OH)(OAc) 有效制备氟烷基化喹啉-2,4-二酮的实用合成方法 2 作为催化剂。该反应始于光驱动的配体到金属电荷转移(LMCT)过程,使反应物脱羧并产生氟烷基自由基,然后与底物进行自由基级联环化。目标产物的收率达到 55-91%。还使用连续流光催化装置完成了克级实验。
更新日期:2024-06-18
中文翻译:

通过铁催化 LMCT 脱羧过程光诱导合成氟烷基喹啉酮
在此,我们报道了一种以 N-(2-氰基苯基)-N-甲基丙烯酰胺为底物、氟烷基羧酸为反应物和 Fe(OH)(OAc) 有效制备氟烷基化喹啉-2,4-二酮的实用合成方法 2 作为催化剂。该反应始于光驱动的配体到金属电荷转移(LMCT)过程,使反应物脱羧并产生氟烷基自由基,然后与底物进行自由基级联环化。目标产物的收率达到 55-91%。还使用连续流光催化装置完成了克级实验。






































 京公网安备 11010802027423号
京公网安备 11010802027423号